Abstract
BACKGROUND AND PURPOSE: Variant Creutzfeldt-Jakob disease (vCJD) is a rare but important cause of dementia and death in young patients and is causally linked to bovine spongiform encephalopathy. Symmetrical hyperintensity in the pulvinar (posterior) nuclei of the thalamus (pulvinar sign) on brain MR images was described as a specific, noninvasive, diagnostic sign of vCJD in a previous small series. This purpose of this larger study was to evaluate this sign prospectively and further define the MR imaging characteristics of vCJD.
METHODS: As part of the ongoing surveillance program in the United Kingdom, MR images of suspected cases of vCJD were collected during a 6-year period. All available images were assessed prospectively by one observer for the presence of the pulvinar sign. Images of neuropathologically confirmed cases were then assessed independently by two neuroradiologists for the degree of hyperintensity of the pulvinar on images of different MR sequences, and for the presence of abnormal hyperintensity in other areas of the brain. Discrepancies were reviewed jointly and a consensus opinion formed.
RESULTS: Prospective analysis identified the pulvinar sign in 74 of 82 cases of vCJD. In the retrospective study, the pulvinar sign, as defined by hyperintensity of the pulvinar relative to the anterior putamen, was present on seven (9%) of 75 T1-weighted, 77 (71%) of 108 T2-weighted, 47 (81%) of 58 proton density-weighted, and 30 (100%) of 30 fluid-attenuated inversion-recovery (FLAIR) images. Diffusion-weighted images were available in two cases and were positive for the pulvinar sign in one. Other features were hyperintensity of the dorsomedial thalamic nuclei (93%), caudate head (40%), and periaqueductal gray matter (83%) on FLAIR images.
CONCLUSION: In the appropriate clinical context, demonstration of the pulvinar sign on MR images is a highly accurate diagnostic sign for vCJD. FLAIR sequence is more sensitive than other sequences. Positive MR images may obviate more invasive diagnostic tests in most cases.
Creutzfeldt-Jakob Disease (CJD) is one of a group of diseases known as the transmissible spongiform encephalopathies. These encephalopathies affect many different animal species and are characterized by a progressive fatal neurologic course and the presence at brain histologic examination of spongiform change, neuronal loss, astrocytosis, and deposition of partially protease-resistant prion protein. This abnormal protein is thought to be the infective agent and is termed a “prion” (1). A number of CJD subtypes are recognized. The most common is sporadic CJD (sCJD), which is found worldwide and has an incidence of about one per million annually. Rarer forms include iatrogenic CJD (transmitted by CJD-infected neurosurgical instruments, or tissue or hormone preparations), and familial CJD (associated with mutations of the prion protein gene). In 1996, a new and clinicopathologically distinct form of CJD was described (2) and named variant CJD (vCJD). Since the identification of the first case in 1995, most patients have been current residents of the United Kingdom, but cases have been recognized in France, the Republic of Ireland, Hong Kong, Italy, Canada, and the United States. A total of 128 definite or probable cases have been documented up to the end of October 2002. vCJD is believed to have occurred as a consequence of the United Kingdom epidemic of bovine spongiform encephalopathy in cattle, with subsequent transmission to humans through infected beef products (3, 4). Although most cases of bovine spongiform encephalopathy have occurred in the United Kingdom, affected cattle have been identified in many countries worldwide. Coupled with the widespread exportation of bovine products, this suggests that the population potentially exposed to the bovine spongiform encephalopathy agent is large and geographically diverse (5).
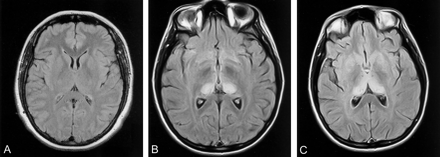
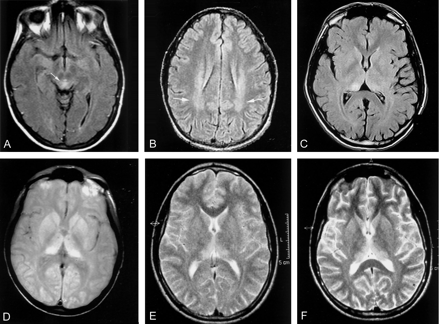
Early and accurate in vivo diagnosis of vCJD is important for prognostic and epidemiologic purposes and for the development of potential therapies. A diagnosis of definite vCJD can be made only by neuropathologic examination, either by in vivo biopsy of brain tissue or at postmortem examination (Table 1). The neuropathologic features defining definite vCJD recently have been described in detail (6). Diagnostic tests useful in sCJD include characteristic electroencephalographic periodic discharges and raised CSF 14–3-3 protein levels. However, these periodic discharges are not seen in vCJD, and CSF 14–3-3 levels are less sensitive in vCJD (7). Brain biopsy and tonsil biopsy provide histologic tissue but are invasive, require general anesthetic, and carry a risk of significant morbidity from the procedure. The presence of characteristic abnormalities on MR images in vCJD cases was first reported in 1997 (8), and subsequent analysis of 36 cases and 57 controls (9) suggested that, in the appropriate clinical context, abnormal high signal intensity in the posterior thalamus (the pulvinar sign, Fig 1B) and in dorsomedial thalamic nuclei (the “hockey-stick” sign, Fig 1C) were sensitive and specific features of vCJD. The aim of the current study was to evaluate prospectively the reliability of the pulvinar sign, as additional cases of vCJD have been identified, and to further define the MR imaging characteristics of vCJD in a larger group of patients.
A, Normal FLAIR image at the level of the basal ganglia shows the thalamus is normally isointense or slightly hypointense relative to the putamen. This appearance is depicted with most sequences, particularly the FLAIR sequence.
B, Pulvinar sign of vCJD. FLAIR image shows marked, symmetrical hyperintensity of the pulvinar (posterior) thalamic nuclei. In this case, the pulvinar signal intensity was scored as grade 4 by both observers.
C, “Hockey-stick” sign of vCJD. FLAIR image shows symmetrical pulvinar and dorsomedial thalamic nuclear hyperintensity. This combination gives a characteristic “hockey-stick” appearance and was present in 93% of cases with FLAIR imaging.
World Health Organization Case Definition for vCJD (6)
Methods
Prospective Study
From 1996 to 2002, all MR images of patients with a clinical history suggestive of CJD (vCJD, sCJD, or other) referred to the United Kingdom National Creutzfeldt-Jakob Disease Surveillance Unit (NCJDSU), Edinburgh, Scotland, were reviewed prospectively by one neuroradiologist (D.A.C.) experienced in the assessment of MR images in CJD. MR images were assessed for the presence or absence of basal ganglia and thalamic hyperintensity, and other features suggestive of any subtype of CJD. This assessment was performed with the neuroradiologist unaware of the clinical diagnosis. The most likely radiologic diagnosis, based on the MR imaging appearances, was recorded in the surveillance database.
Retrospective Analysis
Subsequently, from these examinations, all available MR images from patients with neuropathologically confirmed vCJD were collected for analysis. Where studies were incomplete, or had not been retained at the time of initial referral to the NCJDSU, copies of all MR images were requested from the original referring hospitals. All images were analyzed first independently and subsequently by consensus by two neuroradiologists (D.A.C., D.M.S.).
Imaging Sequences
The imaging examinations were performed in more than 30 hospitals across the United Kingdom, with a variety of MR imaging units. Most commonly, T1-weighted, T2-weighted, and proton density (PD)-weighted sequences were performed. The specific imaging parameters varied considerably by site and manufacturer. Later in the study period, there was increasing use of the fluid-attenuated inversion-recovery (FLAIR) sequence, often replacing the PD-weighted sequence.
Image Quality
Images were subjectively scored for image quality based on the presence and degree of movement artifact and on the quality of the hard-copy images collected (gray-scale contrast based on the ability to distinguish clearly between gray matter and white matter of the cerebral cortex, without excessive hyperintensity in the brightest regions of the image). The gray-scale quality was graded from 1 to 4, with 3 or 4 considered of acceptable diagnostic quality. All images were included in subsequent analysis.
Image Analysis
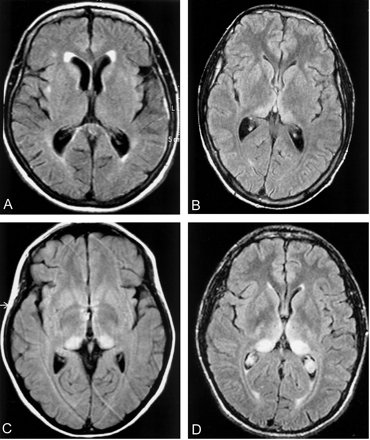
Because digital data were not available for most images (many of which were not Digital Imaging and Communications in Medicine, or DICOM, data), assessment of signal intensities of different brain structures was undertaken by using a reference visual scale: normal white matter was graded 0, and normal gray matter was graded 1. Abnormal hyperintensity relative to normal gray matter was then graded 2–4, representing mild, moderate, or marked hyperintensity, respectively (Fig 2A–D). This was a development of the scoring system used in our previous analysis of MR imaging in vCJD (9).
Grading of degree of pulvinar hyperintensity in vCJD. Because only hard-copy images were available in most cases of vCJD, a 5-point semiquantitative scoring system was used to grade the degree of hyperintensity of the cerebral structures, with white matter scored as 0, normal gray matter scored as 1, and pathologic hyperintensity scored from 2 to 4. Examples of FLAIR images are presented.
A, Normal FLAIR image for comparison.
B, Grade 2 hyperintensity of pulvinar.
C, Grade 3 hyperintensity of pulvinar.
D, Grade 4 hyperintensity of pulvinar
For each imaging sequence, the signal intensities of the cerebral cortex, the caudate head, putamen, globus pallidus, posterior and dorsomedial thalamic nuclei, periaqueductal gray matter, and cerebral white matter were scored by each observer by using the five-point scale described above. Note also was made of the presence and degree of cerebral and/or cerebellar atrophy and of any other unusual features.
Images from each sequence were then scored as positive or negative for the presence of the pulvinar sign. This was defined as bilateral hyperintensity of the pulvinar nuclei of the thalamus relative to the signal intensity of the anterior putamen, which is a modification of the original definition of the sign. We revised the definition as we had identified cases, since our earlier study, of vCJD with abnormal hyperintensity in the caudate head in addition to the pulvinar, and we thought that it was misleading to compare the thalamus and caudate head alone.
The frequency and degree of hyperintensity in the pulvinar, other thalamic nuclei, and other gray and white matter structures were then calculated, based on the consensus opinion.
Interobserver Accuracy
To assess the robustness of the pulvinar sign between different observers, the interobserver variation for scoring of the pulvinar signal intensity and presence of the pulvinar sign for the two independent observers analyzing the images was calculated for each sequence type by using the κ statistic.
Results
Prospective Study
During the 6-year period, 368 MR imaging examinations were collected from patients referred to the NCJDSU, Edinburgh, who had an initial clinical history suspicious for a diagnosis of CJD (variant, sporadic, or other). During this period, all images were reviewed prospectively by one neuroradiologist experienced in the assessment of MR imaging in CJD (D.A.C.). Eighty-two patients with subsequent pathologically confirmed vCJD had images available at the time of initial referral to the NCJDSU. Of these 82 cases, 74 were classified as probable vCJD based on the presence of the pulvinar sign during the prospective analysis, and eight were negative. At subsequent assessment (two neuroradiologists by consensus), one patient previously classified as negative for the pulvinar sign by D.A.C. was reclassified as positive, and two patients initially scored as positive were reclassified as negative. In the remainder of the patients, their original classification remained unchanged. Only two MR imaging examinations with a positive pulvinar sign (confirmed on consensus review) did not fulfill the current clinical criteria for definite or probable vCJD. Both cases were of a fatal dementing illness in young patients, with no other diagnosis made, and for both patients vCJD is still considered the most likely cause “on balance of probability.” No postmortem examination was performed in one case. The second patient was still alive at the time of publication.
Retrospective Study
Of the first 124 cases of vCJD diagnosed up to August 2002, 92 patients had a neuropathologically confirmed diagnosis of definite vCJD. In the remaining 32 cases, the diagnosis of probable vCJD was based on a combination of appropriate clinical history and either MR imaging features or positive tonsil biopsy results, or both. The following analysis refers to the neuropathologically confirmed cases of vCJD only.
Eighty-six of the 92 patients with definite vCJD had images available for review (for four of these patients no images had been reviewed prospectively). In four patients, images could not be retrieved from the original hospital, and in two patients no MR imaging was performed. A total of 110 MR imaging examinations were available in these 86 patients: 65 patients had one image, 19 had two images, and one patient each had three and four images. A total of 273 imaging sequences were assessed (75 T1-weighted, 108 T2-weighted, 58 PD-weighted, 30 FLAIR, and two diffusion-weighted [DW] sequences).
Image Quality
Most images showed no significant motion artifact or only minor motion artifact (with most obtained without general anesthetic). Twenty-eight (25%) of 110 studies were graded as showing moderate or severe motion artifact. Twenty-four of these studies (86%) were positive for the pulvinar sign. In several cases with very marked movement artifact, the pulvinar sign was still clearly visible.
Assessment of the effect of image contrast on the diagnostic quality of examinations was difficult owing to the wide variation in a number of imaging factors. Image gray scale and contrast depends on several parameters, including the type and age of the imaging unit, sequence parameters, image window level and width, and filming technique. There is intrinsic variation in the conspicuity of gray and white matter with different sequences, so scoring was standardized to reference images of that particular sequence, ranging from 1 (no differentiation) to 4 (excellent differentiation). Positive T2-weighted images had a mean gray-scale score of 2.84 (range, 1–4; n = 95), whereas negative T2-weighted images had a mean score of 2.23 (range, 1–4; n = 13), indicating that the quality of image contrast was poorer in negative cases.
Independent Review
When viewed independently, observer 1 scored 91 (83%) of 110 examinations as positive for the pulvinar sign, and observer 2 score 100 (91%) of 110 examinations as positive. Interobserver agreement κ scores were good for T2-weighted and PD-weighted images and showed complete concordance for FLAIR imaging (Table 2)
Variation in Interobserver Agreement for the Presence of the Pulvinar Sign on MR Images in 86 Cases of vCJD
Consensus Review
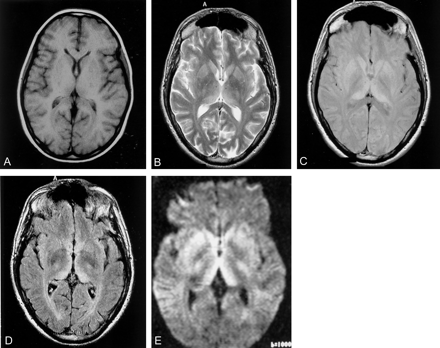
The number of images from each sequence considered positive by consensus from these examinations is shown in Table 3. T1-weighted images were the least sensitive, and no contrast material enhancement of the basal ganglia was seen in the nine cases with gadolinium-enhanced T1-weighted sequences. FLAIR was the most sensitive sequence for detecting the pulvinar sign (Fig 3A–D). DW images were available only in two cases, one of which showed marked pulvinar hyperintensity. In addition, this DW imaging study showed generalized hyperintensity of the caudate head and putamen (Fig 3E).
Effect of MR sequence on visibility of the pulvinar sign.
A, Nonenhanced T1-weighted image. High signal intensity was relatively rarely detected on T1-weighted images, which was thought to be secondary to the T1-shortening effect from marked prion protein deposition. Contrast enhancement was not seen in any case.
B–D, The pulvinar sign was more easily seen on T2-weighted (B) and PD-weighted (C) images, but was clearly visible on FLAIR (D) images.
E, DW image was also positive in one of two available cases.
Frequency of MR Images with a Positive Pulvinar Sign in Patients with vCJD
Seventy-eight (91%) of 86 patients had at least one MR imaging examination positive for the pulvinar sign at consensus review. Using all available sequences for each MR examination, 96 (87.2%) of 110 examinations were considered positive for the pulvinar sign on images of one or more sequences at consensus review.
Cases of vCJD with Multiple MR Examinations
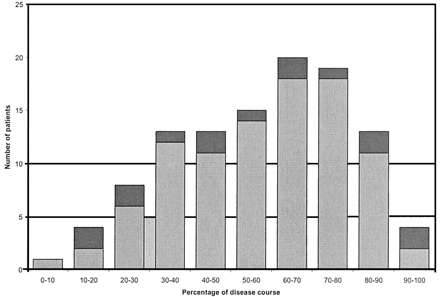
Of the 21 patients with two or more MR examinations, in 18 cases (86%) the first image was positive for the pulvinar sign and in two cases all the images were considered negative. One of these latter patients had a very limited examination; the other had two high-quality MR examinations that were unequivocally negative. One patient had two negative images followed by a positive image. The first two images in this individual were very limited studies. Three patients (14%) who had an initially positive image subsequently had a negative MR imaging examination later in their illness. These later images were, however, rated as of nondiagnostic quality (gray-scale quality 1 or 2) in two of the three MR imaging examinations. Six patients underwent imaging within 100 days of first recorded symptom onset, as defined retrospectively from the clinical data. All six of these examinations were positive. Negative MR imaging examinations occurred at all stages in the disease course (Fig 4). MR images positive for the pulvinar sign were obtained at a mean of 58% (95 examinations) of the illness course (from first symptom to death). Negative MR images were obtained at a mean of 55% (15 examinations) of the disease course.
Graph shows timing of MR imaging in relation to vCJD duration. Dark gray indicates negative MR images; light gray, positive MR images
The degree of hyperintensity of the pulvinar varied among sequences, with 32 (30%) of 108 T2-weighted images showing moderate or marked pulvinar hyperintensity (scored 3+), 35 (60%) of 58 PD-weighted images, and 24 (80%) of 30 FLAIR images.
The presence and degree of hyperintensity of the other deep gray matter structures also varied with sequence weighting (Table 4). Common additional findings included dorsomedial thalamic nuclei hyperintensity, periaqueductal gray matter hyperintensity (Fig 5A), and hyperintensity of the caudate head. Dorsomedial thalamic hyperintensity was seen on most FLAIR images, giving a very characteristic “hockey-stick” distribution of hyperintensity.
Other MR imaging findings in vCJD.
A, Axial FLAIR image shows periaqueductal gray matter hyperintensity (arrow). Though not a specific sign, periaqueductal gray matter hyperintensity was seen in 83% of cases with FLAIR imaging.
B, FLAIR image shows abnormal hyperintensity in the centrum semiovale white matter (arrows), reflecting the diffuse involvement of the brain by the disease.
C, FLAIR image shows asymmetrical bilateral pulvinar hyperintensity. Asymmetrical hyperintensity was a rare finding in vCJD, seen in less than 5% of cases.
D, PD-weighted image shows widespread basal ganglia hyperintensity. Though hyperintensity in the basal ganglia is also seen in a number of other conditions, the signal intensity of the pulvinar remains the most hyperintense, helping to categorize this case correctly as probable vCJD.
E and F, Case of progressive cerebral atrophy in vCJD. Two images taken 3 months apart show subtle but definite global cerebral atrophy. Unlike in sCJD, cerebral atrophy is not a prominent feature of vCJD and is most easily seen in the parietooccipital region.
Other MR Abnormalities in Deep Gray Matter Structures in vCJD
A number of less common MR imaging findings were also noted. Abnormal hyperintensity of the parietooccipital white matter (grade 2 or greater) was seen on 13 (20%) of 65 T2-weighted images and 11 (37%) of 30 FLAIR images (Fig 5B). In three cases, significant asymmetry of pulvinar hyperintensity was noted (Fig 5C). Cerebral atrophy was an uncommon feature, with cerebral atrophy judged as moderate or severe for age noted in 13 (15%) of the 86 patients. Generalized cerebellar atrophy was seen in eight patients (9%). In two of 21 cases with serial imaging, there was a significant subjective progression in cerebral atrophy over the interval between images in these subjects (interval 67 and 144 days, respectively) (Fig 5E and F).
Discussion
Our results confirm that the pulvinar sign is a reliable and accurate diagnostic test in cases of clinically suspected vCJD. The FLAIR sequence is more sensitive than either T2-weighted or PD-weighted imaging for detection of the pulvinar sign. To our knowledge, this is the largest study of the MR imaging appearances of vCJD. It confirms that the inclusion of characteristic features on MR images in the World Health Organization (WHO) criteria for the diagnosis of vCJD is appropriate. The presence of the pulvinar sign on brain MR images is the most accurate noninvasive diagnostic test of vCJD. The current WHO diagnostic criteria and categories for vCJD are listed in Table 1.
Based on recent experience, we recommend slight modification of the definition of the pulvinar sign. Recognition of the sign depends on comparing the signal intensity of the pulvinar to that of other cortical and deep gray matter structures. The pulvinar sign was originally defined simply as hyperintensity of the pulvinar. With experience, however, this definition is noted to be less specific than originally considered for a number of reasons: In young patients, the normal basal ganglia are relatively hyperintense compared with basal ganglia signal intensity in the older population on long TR sequences, notably PD-weighted images, and this may mimic MR imaging changes of CJD. (The age-related decrease in basal ganglia signal intensity is probably secondary to progressive iron deposition over life [10].) Also, cases of sCJD in young patients have been described with hyperintensity in the pulvinar relative to the other thalamic nuclei, making the pulvinar more conspicuous, and this could be mistaken for vCJD, although in these sCJD cases, the signal intensity of the pulvinar always remains less than that of the anterior putamen (Fig 5D) (11). In addition, in a few cases of vCJD, marked hyperintensity is seen in the caudate head, and this may match the signal intensity in the pulvinar, resulting in a false-negative image based on the original criteria. For the above reasons, we recommend the pulvinar sign be redefined as hyperintensity of the pulvinar relative to the signal intensity of the anterior putamen. The pulvinar hyperintensity in vCJD is usually symmetrical, and marked asymmetry should bring the diagnosis into question, with only three examinations in this series showing significant asymmetry.
Some other features, such as the presence of abnormal hyperintensity in the periaqueductal gray matter and posterior deep white matter tracts, were also common, and although signal intensity changes in these sites were more difficult to perceive, and are less specific, they may be useful corroborative features in difficult cases. However, the presence of hyperintensity in the dorsomedial nuclei of the thalamus on FLAIR images is a helpful feature, with a sensitivity approaching that of the pulvinar sign.
Our study is open to a number of criticisms. The need to assess hard-copy images because of the lack of digital data introduces a number of uncontrolled variables in image quality due to differences in imager manufacturer, sequence selection, and most importantly the window settings selected for imaging by the MR technician. The MR hard-copy images available for this study were of highly variable quality, as they were acquired as routine clinical investigations in over 30 imaging centers across the United Kingdom, on MR imaging units of varying age and field strength, with varying sequences, imaging planes, gray-scale presentation, and movement artifact. Movement artifact does not appear to hinder diagnosis significantly, but poor gray-scale contrast was more often seen with the negative images and may be a contributory factor to the number of false-negative images. This study has shown, however, that despite the wide variation in image quality, subjective qualitative analysis of the pulvinar sign is a robust method of diagnosing vCJD.
Although no control group was used in the retrospective part of the current study, in our previous study (9) we compared vCJD with sCJD and controls in a blinded fashion and found very similar sensitivities for the pulvinar sign to this study for PD- and T2-weighted images. We have now shown that FLAIR imaging is more sensitive than either of these two sequences. Identifying an adequate size control group of MR images in age-matched normal cases or in cases of clinically suspect CJD (but subsequently proved normal) is difficult, and given the relative rarity of the condition, the frequency of vCJD cases will always be overrepresented in any retrospective sample. However, in the prospective analysis of all 368 examinations assessed over the 6-year period of data collection viewed with the clinical data unknown to the assessor (D.A.C.), there was very close correlation in the number of definite cases of vCJD correctly categorized as positive at first viewing and at subsequent review (79 of 82 cases correctly classified as positive on both viewings by D.A.C.). This suggests that the pulvinar sign is reliable. A large, international, multiobserver study assessing the pulvinar and other MR imaging signs in CJD is currently in preparation. It is hoped that this will further clarify the role of MR imaging and define the optimal imaging protocol to diagnose and differentiate the various forms of CJD.
Hyperintensity involving the whole thalamus or focal involvement anteriorly with sparing of the posterior nuclei has been described in a number of conditions, but in these cases the distribution of hyperintensity is usually distinct from that seen in vCJD. In a young patient, the main clinical differential diagnosis of vCJD is sCJD. As discussed above, although occasionally the pulvinar and dorsomedial nuclei are hyperintense relative to the other thalamic nuclei in sCJD, in the 368 images reviewed through our unit we have not seen any case of sCJD in which the signal intensity of the pulvinar was higher than that of the anterior putamen, and this has remained the robust radiologic feature of the pulvinar sign that discriminates vCJD from sCJD.
Previous studies of the distribution of the histologic changes in vCJD show vacuolation, neuronal loss, and astrocytosis in several posterior thalamic nuclei, but the pulvinar shows a much higher degree of neuronal loss and astrocytosis than these other nuclei and indicates that the histologic substrate of the pulvinar sign is most likely astrocytosis. MR imaging studies in experimental scrapie also indicate that the pathologic substrate of high signal intensity in the brain is astrocytosis (12). The involvement of the thalamus may reflect the hypothetical route of access of the infective agent into the CNS via the ascending pathways, which relay at the thalamus. It is of interest to note that the periaqueductal gray matter also exhibits severe astrocytosis in vCJD (Prof J Ironside, personal communication, 2002). Astrocytosis is considered to be an irreversible process, reflecting a response to tissue damage within the CNS. It is therefore considered unlikely that on the long TR sequences under examination the pulvinar sign would disappear as a result of treatment of prion diseases. It also suggests that by the time the pulvinar sign is positive, the disease is advanced. Further studies are needed to assess the radiologic-neuropathologic correlates in other nuclei in vCJD, in sCJD, and in other forms of CJD.
This study has confirmed that T1-weighted sequences are least likely to show significant abnormality. Hyperintensity on T1-weighted images has been described in the globus pallidus in patients with sCJD (13), and results of both human and animal studies suggest that hyperintensity on T1-weighted images is due to dense depositions of the abnormal form of prion protein. T2-weighted sequences are more sensitive, although partial volume artifact from the high-signal-intensity CSF in the quadrigeminal cistern has been found to be misleading in a number of cases.
Pulvinar hyperintensity is often more conspicuous on the PD-weighted images than on T2-weighted images, and these were found to be the most useful images in the early cases assessed previously. However, we have become aware of PD-weighted sequences on some modern MR imagers that may show the putamen and caudate heads as hyperintense relative to the cerebral cortex in neurologically normal subjects, and this may be a confounding factor. In this study, the anterior basal ganglia were perceived as hyperintense more commonly on PD-weighted images than on either T2-weighted or FLAIR images. Further work is required to validate this against an appropriate control group. In the interim, we recommend caution when assessing the pulvinar sign on PD-weighted images.
In our study, FLAIR imaging was 100% sensitive to detecting the pulvinar sign in vCJD, with 83.3% showing moderate or marked hyperintensity of the pulvinar nuclei and with 93% also having abnormal hyperintensity in the dorsomedial nuclei. We had previously described the high sensitivity of FLAIR imaging in vCJD diagnosis (14), but were surprised to find the remarkable consistency of this finding. However, subsequently we have seen at least one case currently classified as probable vCJD with normal FLAIR images. Neuropathologic confirmation of the definitive diagnosis in this case is awaited. FLAIR sequences have also been shown to be useful in sCJD (15). Unlike in sCJD, cortical signal intensity change on FLAIR images was rarely identified in vCJD. The reason for this is unknown, but may relate to differences between the two diseases in the stage or extent of disease at the time the diagnosis is suspected clinically, or of differing modes of entry into or spread within the central nervous system.
The number of DW images available for analysis in this study was disappointingly small. This was largely because most cases with neuropathologic confirmation were diagnosed between 1996 and 2000, when DW imaging was not widely available or used routinely in most centers in the United Kingdom. DW imaging has been reported to be highly sensitive to hyperintensity of the putamen and caudate early in the clinical course of sCJD (16), and it is possible that pulvinar and dorsomedial thalamic signal intensity changes on DW images may prove to be equally useful in vCJD. However, it has also been noted in sCJD that DW imaging hyperintensity may disappear later in the disease (16), and therefore the exact role of DW imaging in vCJD remains to be determined.
Pulvinar hyperintensity was present on the first examination in over 85% of cases with serial imaging and was positive in one patient within 2 months of the onset of symptoms In addition, in only one patient did the image change from negative to positive, suggesting that the pulvinar sign becomes positive early in relation to the time neurologic disease is suspected clinically. It remains unknown whether the pulvinar sign is positive in the presymptomatic stage of the disease.
We have encountered a number of cases in which the pulvinar sign has been overlooked or overdiagnosed. In the first 32 cases, the abnormality was overlooked in the initial radiology report in two-thirds of patients. The frequency of false-negative reporting has fallen as awareness of the pulvinar sign by radiologists in the United Kingdom has increased, but the sign is still occasionally overlooked, often because no images are acquired in the appropriate axial plane (anterior commissure-posterior commissure line) to allow direct comparison of basal ganglia and thalamic signal intensity on the same section. For this reason, we advise that all MR images in suspected cases be reviewed by neuroradiologists with experience in the assessment of MR images in the different subtypes of CJD.
Conclusion
This study has confirmed that the pulvinar sign is a robust diagnostic sign of vCJD. MR imaging is the imaging investigation of choice in clinically suspected vCJD, and a positive pulvinar sign allows the diagnosis of probable vCJD to be made, without the need for further investigation in most cases. However, to maintain the highest diagnostic accuracy of MR imaging in vCJD, we recommend that the pulvinar sign should be recorded as “present” if 1) there is an appropriate clinical history, according to the primary criteria for case definition (Table 1); 2) there is bilateral hyperintensity in the pulvinar, greater than the signal intensity in the anterior half of the putamen; and 3) appropriate imaging sequences have been performed, preferably including a FLAIR sequence in the axial plane.
Acknowledgments
We thank the staff of the United Kingdom National Creutzfeldt-Jakob Disease Surveillance Unit for data collection and clinical support. We also thank all clinicians who have collaborated with United Kingdom CJD surveillance project, which has facilitated this work.
Footnotes
Supported by the United Kingdom Department of Health grant no.121–7369.
References
- Received December 12, 2002.
- Accepted after revision March 4, 2003.
- Copyright © American Society of Neuroradiology