Abstract
BACKGROUND: A shift has occurred in interventional cardiology from transfemoral to transradial access due to a 70%–80% decrease in complications. This shift has not yet taken place in other interventional specialties, perhaps owing to the lack of generalizability of findings in the cardiology data.
PURPOSE: Our aim was to assess data from the recent mechanical thrombectomy prospective trials to better understand the access-site complication rate.
DATA SOURCES: Articles were systematically sourced from the National Center for Biotechnology Information PubMed archive.
STUDY SELECTION: According to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis guidelines, prospective, randomized controlled trials published after 2008 with mention of major and/or minor femoral access-site complications in neuroendovascular mechanical thrombectomies were included.
DATA ANALYSIS: Major and minor femoral access-site complications were extracted. A total complication rate was calculated with major access-site complications alone and combined with minor access-site complications.
DATA SYNTHESIS: Seven prospective studies of 339 total screened met the inclusion criteria. Eleven major access-site complications were identified in of 660 total interventions, revealing a major access-site complication rate of 1.67% for patients undergoing mechanical thrombectomy with transfemoral access. If minor access-site complications were included, 35 total incidents were detected in 763 interventions, resulting in a total complication rate of 4.59%.
LIMITATIONS: Multiple unspecified vessel and procedure-related complications were mentioned in the studies.
CONCLUSIONS: The overall rate of major access-site complications was 1.67% in this review, which is not low and poses a risk to patients. We suggest further investigation into the feasibility and complication rates of alternative access sites for neurointerventional procedures.
ABBREVIATION:
- PRISMA
- Preferred Reporting Items for Systematic Reviews and Meta-Analysis
The field of interventional cardiology in the United States and internationally has shifted away from transfemoral access to transradial access, given the profound safety benefits, including a remarkable reduction in access-site complications such as major/minor bleeding, pseudoaneurysm, and hematoma development.1⇓⇓⇓⇓⇓-7 Transradial access also leads to earlier ambulation postoperatively, shorter hospital stays, reduced costs, and improved patient satisfaction.5,6 Furthermore, successful transradial access has been reported in cases of failed transfemoral access secondary to tortuosity, stenosis near the aortic arch, bilateral iliac occlusions, and aortic dissection.8 Despite numerous prospective, randomized trials in the interventional cardiology literature, a shift away from transfemoral access toward transradial access in neurointerventional surgery has not yet been realized, with only 0.3%–4.5% of patients undergoing thrombectomy having transradial access in cerebrovascular interventions.9
Multiple reasons behind this slower adoption include the learning curve associated with accessing the cerebrovasculature via transradial access10 and anatomic variants complicating radial access with failure to reach the anterior cerebral vasculature, reported to be due to proximal left common carotid and right subclavian tortuosity, while failure to catheterize the vertebral arteries has been reported due to acute angulation and proximal origin of the vertebral arteries.11 Other reasons for the slower adoption include difficulty accessing the cerebrovasculature using current transfemoral devices and a perceived lack of transfemoral-access complications during neurointerventional procedures.
Furthermore, there is the question of whether the wealth of transfemoral access data from interventional cardiology is generalizable to our specialty, owing to differences in anticoagulation regimens, procedural type, and access and hemostasis regimens. For example, in cardiology, 6F is the largest diameter catheter that could be effectively used via the transradial access.12 Prior studies in animal models have shown that the minimal inner-catheter diameter needed for successful thrombectomy of the middle cerebral or internal carotid arteries is >0.040 inches and >0.064 inches, respectively, thus presenting a limitation in the minimum catheter size with which thrombectomy can be effectively performed via transradial access.13
We sought to obtain an objective understanding of transfemoral access-site complications in our own field and performed a systematic review of the prospective trial data regarding mechanical thrombectomy.
MATERIALS AND METHODS
Search and Information Sources
This review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The PRISMA statement consists of a 27-item checklist and a 4-phase flow diagram.14 The aim of the PRISMA statement is to help authors improve the reporting of systematic reviews and meta-analyses. In addition to the PRISMA statement, a supporting explanation and elaboration document has been produced following the style used for other reporting guidelines.15
Articles were sourced from the National Center for Biotechnology Information PubMed archive, the New England Journal of Medicine, Stroke, and Lancet Neurology. The search terminology entered into the PubMed archive included “mechanical thrombectomy + prospective OR mechanical thrombectomy + RCT,” to locate the specific articles analyzed in this review. Articles considered for the review were only those published from 2008 to 2018.
Eligibility Criteria and Study Selection
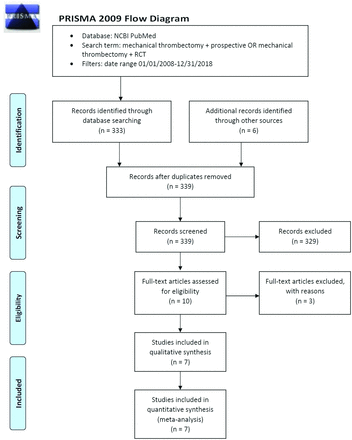
Articles included in this review had to meet the following criteria: prospective, randomized controlled trials. Studies that did not specifically identify groin or access-site complications were deemed ineligible, including several large transfemoral thrombectomy trials such as A Direct Aspiration, First Pass Technique (ADAPT), Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN), and Solitaire With the Intention For Thrombectomy as Primary Endovascular Treatment (SWIFT PRIME), and were excluded from this review because these studies failed to identify access-site complications specifically, instead grouping them under overall procedural complications. Accordingly, 3 full-text articles that met the initial screening criteria were subsequently excluded.16⇓-18 Serious transfemoral access-site complications were assessed in mechanical thrombectomies during an acute ischemic stroke. In the context of the included articles, serious complications/adverse events are defined as complications that meet any of the following criteria: resulted in a >3-g hemoglobin or a 10% hematocrit drop, required surgical/interventional radiology intervention, required transfusion, prolonged the patient’s stay in the hospital, or resulted in death. Examples include groin hematoma requiring transfusion, artery dissection, pseudoaneurysm, and occlusion requiring embolectomy. Studies that addressed only minor access-site complications (ie, access-site ecchymosis) were excluded. Any studies using nonfemoral access-sites, written in a language other than English, and written before 2008 were also excluded (Figure).
PRISMA flowchart.
Data Collection Process
Articles were compiled into a single data base from which identical and irrelevant articles were removed. Of the remaining articles, 7 articles met the inclusion criteria.19⇓⇓⇓⇓⇓-25 The 7 publications included were critically evaluated by the authors, and access-site complication rates (major, minor, and total) were extracted and compiled into a single databank.
RESULTS
Individual Study Characteristics
The methodology for each clinical trial is summarized in Table 1. Of note, the studies differed in device use for mechanical thrombectomy, timing of intervention, location of vessel occlusion, and tPA administration.
Summary of clinical trial methodology
Data Analysis
The access-site complication rates for each of the studies (Table 2) ranged from 0% to 11.65%. Access-site complication rates were calculated by dividing the total number of access-site complications by the total number of participants in the mechanical thrombectomy arm of each study. The access-site complication rate, including both major and minor adverse events, gleaned from pooled data was 35/763 (4.59%). Subgroup analysis revealed that 11 major access-site complications were identified of 660 total interventions, revealing a major access-site complication rate of 1.67% for patients undergoing mechanical thrombectomy with transfemoral access.
Access-site complication rates
There is mention of vessel dissections and perforations in these studies; however, the vessel was unspecified in all cases.
DISCUSSION
The clinical efficacy of mechanical thrombectomy in the management of acute ischemic stroke has been investigated in numerous randomized controlled trials. While the benefits and indications of mechanical thrombectomy continue to unfold, there is a paucity of research into the access-site-associated complications from these procedures.
Prior retrospective series likely underreported the rate of transfemoral access-site complications26 because these studies may not include major, non-life-threatening complications. Thus, our current understanding of access-site complications is limited, given the inherent limitations of retrospective review. This study sought to use high-level evidence to more accurately estimate the incidence of transfemoral access-site complications.
Our review found that the rate of serious access-site complications related to transfemoral access in mechanical thrombectomy was 1.67%, demonstrating that adverse events occurred in a notable percentage of transfemoral access stroke interventions. It is quite possible that the true rate of adverse events in our review was even greater than the reported figure because there were a number of adverse events that may have been access-site-related but could not be confirmed due to ambiguity in adverse event reporting in supplementary appendices.
Our findings are similar to meta-analyses on transfemoral access-site complications in interventional cardiology, which range from 2.2% to 4.8%.27 Despite the technical differences between transfemoral access in interventional cardiology and stroke interventions, such as anticoagulation regimens, procedures, procedure lengths, and access/closure techniques and devices, access-site complication rates are similar. This similarity suggests that the access-site itself, as the consistent factor between the 2 interventions, may play a larger role in the development of complications than expected and that these complications are, in essence, specialty agnostic.
In evaluating the limitations of our included trials, it is pertinent to differentiate major and minor access-site complications. Major access-site complications are defined as any complication that either requires further surgical intervention or prolongs the patient’s hospital stay, consistent with definitions in most stroke trials. These major access-site complications include groin hematoma requiring transfusion and arterial dissection. Minor access-site complications are defined as complications that do not meet major criteria but were recorded in the trials. The minor access-site complications recorded in the studies were ecchymoses, local infection, and minor self-limiting femoral hematomas. Although our review sought to identify and report these major and minor access-site complications, all included studies except 1 (Endovascular Treatment for Small Core and Proximal Occlusion Ischemic Stroke [ESCAPE])20 did not report minor complications. Lack of routine postprocedural sonography may have contributed to the underreporting of these complications.
When reporting vascular complications, many of the included studies did not specify a vessel. This vessel could be the femoral artery, and this would increase the access-site complication rate. Conversely, vessel complications in the cerebral vasculature would decrease the major-site-associated adverse event rate. Furthermore, there are complications listed in supplementary indices that are vague. Some of these include “arterial perforation,” “vessel occlusion,” and “vessel dissection.” These complications may relate to the access-site; however, we could not be sure.
Last, the 4 studies that were excluded during eligibility assessment of access-site-associated adverse events may have altered the adverse event rate if details regarding these events were appropriately reported. Specifically, the Solitaire Flow Restoration Thrombectomy for Acute Revascularization (STAR) and Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trials cited procedure-related adverse events and vessel dissections, and Endovascular Therapy Following Imaging Evaluation for Ischemic Stroke 3 (DEFUSE 3) reported vascular disorders and administration-site conditions. As mentioned in the above paragraph, failure to further subclassify vessel dissections further obfuscated the relation of the complication to the procedural access site.
CONCLUSIONS
The rate of major access-site complications following a transfemoral approach has not been investigated in the context of neurointerventional procedures in prior studies. Our analysis demonstrates rates of major access-site complications from transfemoral access, similar to those reported in the cardiology literature and that may, in fact, be higher. However, transradial access is not without its limitations. The radial artery has a small diameter, which presents clear challenges when introducing the 8F catheter required for cerebrovascular thrombectomies. We suggest further investigation into the feasibility and complication rates of alternative access sites for neurointerventional procedures. Furthermore, given the benefits of a transradial approach, there is a clear need for radial artery–specific cerebrovascular catheters, which are both compatible with radial access while also permitting successful cerebrovascular interventions.
Acknowledgments
The authors acknowledge Vivian R. Hagerty and Hunter R. Carlock for their assistance in writing this article.
Footnotes
Disclosures: Brian M. Snelling—UNRELATED: Stock/Stock Options: RIST Neurovascular, Comments: preclinical neurovascular medical device company, no conflict related to submitted article.
References
- Received September 22, 2019.
- Accepted after revision January 7, 2020.
- © 2020 by American Journal of Neuroradiology