Abstract
BACKGROUND AND PURPOSE: Parry-Romberg syndrome is a rare disorder characterized by progressive hemifacial atrophy. Concomitant brain abnormalities have been reported, frequently resulting in epilepsy, but the frequency and spectrum of brain involvement are not well-established. This study aimed to characterize brain abnormalities in Parry-Romberg syndrome and their association with epilepsy.
MATERIALS AND METHODS: This is a single-center, retrospective review of patients with a clinical diagnosis of Parry-Romberg syndrome and brain MR imaging. The degree of unilateral hemispheric atrophy, white matter disease, microhemorrhage, and leptomeningeal enhancement was graded as none, mild, moderate, or severe. Other abnormalities were qualitatively reported. Findings were considered potentially Parry-Romberg syndrome–related when occurring asymmetrically on the side affected by Parry-Romberg syndrome.
RESULTS: Of 80 patients, 48 (60%) had brain abnormalities identified on MR imaging, with 26 (32%) having abnormalities localized to the side of the hemifacial atrophy. Sixteen (20%) had epilepsy. MR imaging brain abnormalities were more common in the epilepsy group (100% versus 48%, P < .001) and were more frequently present ipsilateral to the hemifacial atrophy in patients with epilepsy (81% versus 20%, P < .001). Asymmetric white matter disease was the predominant finding in patients with (88%) and without (23%) epilepsy. White matter disease and hemispheric atrophy had a higher frequency and severity in patients with epilepsy (P < .001). Microhemorrhage was also more frequent in the epilepsy group (P = .015).
CONCLUSIONS: Ipsilateral MR imaging brain abnormalities are common in patients with Parry-Romberg syndrome, with a higher frequency and greater severity in those with epilepsy. The most common findings in both groups are white matter disease and hemispheric atrophy, both presenting with greater severity in patients with epilepsy.
ABBREVIATIONS:
- ASM
- antiseizure medication
- ECDS
- en coup de sabre
- EEG
- electroencephalogram
- IQR
- interquartile range
- PRS
- Parry-Romberg syndrome
- WMD
- white matter disease
Parry-Romberg syndrome (PRS) is a rare, self-limiting disorder characterized by progressive hemifacial atrophy affecting the skin and subcutaneous tissues. There is also variable involvement of underlying muscle and bone, with occasional involvement of the ipsilateral limbs or bifacial atrophy. It is difficult to differentiate PRS and en coup de sabre (ECDS), or localized scleroderma, due to overlap in the facial features present in both entities.1 The pathogenesis of PRS remains poorly understood, with many proposed theories of the etiology ranging from infection, trauma, vascular abnormalities, autonomic dysregulation, or an autoimmune process, the latter being the leading hypothesis.2,3 PRS occurs more commonly in females, arising in childhood or young adulthood, with eventual stabilization of atrophic features. Once stabilized, patients often undergo reconstructive surgeries and additional treatments depending on the severity of PRS.4⇓⇓⇓⇓-9
Patients with PRS often exhibit neurologic symptoms, including headaches, seizures, and hemiplegia.3,10,11 Intracranial abnormalities on neuroimaging have been previously reported, yet the timing of the development of these intracranial findings with that of the clinical features of PRS has often been inconsistent.10,12,13 Because seizures are a common debilitating morbidity that affects the prognosis of PRS, further characterization of this association is needed to improve diagnosis and management. This study aimed to describe the frequency and severity of MR imaging brain abnormalities in patients with PRS and determine their association with epilepsy.
MATERIALS AND METHODS
Patient Selection and Data Collection
This retrospective, cross-sectional study was approved by the institutional review board. A medical record review was performed to identify patients diagnosed with PRS or hemifacial atrophy from 2000 to 2020 at a multisite tertiary care center. Inclusion criteria were the following: 1) a working clinical diagnosis of PRS, and 2) available MR imaging of the brain consisting of, at minimum, standard structural imaging sequences with T1-, T2-, FLAIR, diffusion, and gradient echo/T2*/susceptibility-weighted images obtained as part of the diagnostic evaluation. Clinical data were collected from the medical records, including patient age at the time of the PRS work-up and MR imaging, age at which hemifacial atrophy first became evident, sex, MR imaging results, clinical and neurologic manifestations, laterality of hemifacial atrophy, and the presence or absence of epilepsy. For patients with epilepsy, documented data included age of onset, seizure semiology, MR imaging, electroencephalogram (EEG), seizure/epilepsy classification, and medical or surgical management.
Imaging Analysis
Prior MR imaging reports were reviewed, and MR imaging studies were further analyzed separately by 2 board-certified neuroradiologists for the presence of brain abnormalities. The main findings studied were hemispheric atrophy, white matter disease (WMD), microhemorrhage, and leptomeningeal enhancement. When present, atrophy, WMD, and microhemorrhage were graded as none, mild, moderate, or severe adapted from scales of global cortical atrophy14 and WMD severity15 established in neurodegenerative and vascular disease (Fig 1). Microhemorrhage and calcification were based on the assessment of susceptibility-weighted imaging and/or gradient echo/multiecho gradient echo (quantitative susceptibility mapping) and verified on CT when available. Brain abnormalities were considered potentially related to PRS when occurring asymmetrically on the side ipsilateral to the hemifacial atrophy. Among patients with bilateral brain involvement of the predefined abnormalities, only conditions of patients with asymmetric hemisphere involvement on the same side as craniofacial changes were counted as likely related to PRS. The side with the highest grade (ie, moderate or severe) was reported as dominant in cases identified with bilateral brain involvement, while the hemisphere with mild involvement was not considered as the lateralized abnormality for statistical measure. The presence of additional brain abnormalities was qualitatively reported.
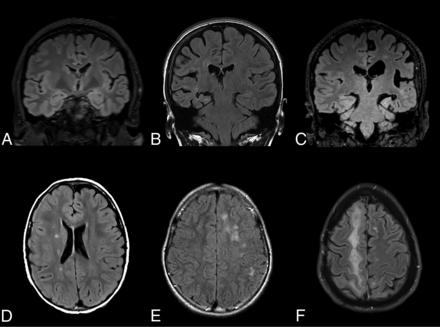
Exemplary MR imaging of atrophy and white matter disease severity. Coronal FLAIR MR imaging shows mild left hemisphere atrophy (A), moderate right hemisphere atrophy (B), and severe left hemisphere atrophy (C). Axial FLAIR MR imaging shows mild right hemisphere WMD (D), moderate left hemisphere WMD (E), and severe right hemisphere WMD (F).
Statistical Analysis
Descriptive analysis was undertaken for quantitative and qualitative variables, which are reported as median (interquartile range [IQR], 25–75) and frequency (percentage), respectively. Categorical variables were compared using the χ2 or Fischer exact test, as appropriate. Statistical analysis was performed using SPSS, Version 25.0 for Windows (IBM) with P < .05 as the significance level.
RESULTS
A total of 80 patients with a working or established clinical diagnosis of PRS and available neuroimaging with brain MR imaging were identified (Table 1). The median age at work-up was 37 years (IQR, 26–56), and most patients were female (70%). Hemifacial involvement was left-sided in 43 (54%) cases. There were 48 (60%) patients with a diagnosis of PRS and brain abnormalities on MR imaging. Of these, 46 (57%) had at least 1 of the predefined abnormalities (ie, hemispheric atrophy, WMD, leptomeningeal enhancement, or microhemorrhages), while 1 patient per cohort had additional, bilateral, intracranial findings only. Thirty-two (40%) patients had an asymmetric hemispheric predominance of brain abnormalities on MR imaging, of whom 26 (32%) patients had abnormalities localized ipsilateral to the hemifacial atrophy. Six (7%) patients had a contralateral predominance of brain abnormalities, and 15 (18%) had bilateral MR imaging brain abnormalities.
Parry-Romberg syndrome patient characteristicsa
Sixteen (20%) patients with a clinical diagnosis of PRS had epilepsy, with a median age of seizure onset of 13 years (IQR, 10–16), as detailed in the Online Supplemental Data. The median number of seizures per month was 2, ranging from 1 every 3 months to 60 seizures per month. Seven (44%) reported a single semiology, while 9 (56%) had >1 type of seizure during their course of illness. Most (81%) experienced seizures with motor symptomatology. Focal aware seizures were seen in 6 (37%) patients with epilepsy; focal impaired awareness seizures, in 9 (56%); and 11 (69%) patients with epilepsy had focal-to-bilateral tonic-clonic seizures. Fourteen (87%) patients were managed with antiseizure medications (ASMs) only, with a median of 2 (range, 1–4) ASMs; 8 (50%) were controlled with a single ASM per last follow-up. Three (19%) patients were drug-resistant, with 2 (13%) receiving vagus nerve stimulation. EEG findings were abnormal in 13 (81%) patients with epilepsy. Eight (50%) had interictal epileptiform discharges on EEG, 6 (37%) arising ipsilateral to both the MR imaging brain and hemifacial findings, 1 (6%) ipsilateral to the MR imaging brain abnormalities yet contralateral to the hemifacial atrophy, and 1 (6%) patient had bilateral epileptiform discharges and bilateral brain abnormalities. Notably, 9 (56%) patients had video EEGs, of whom, 5 (31%) had interictal epileptiform discharges which arose ipsilateral to the hemifacial atrophy in 4 (25%) of patients.
MR imaging revealed brain abnormalities in all 16 (100%) patients with epilepsy compared with 31 (48%) patients without epilepsy (P < .001), of whom, 15 (94%) and 30 (47%), respectively, had at least 1 primary feature (ie, hemispheric atrophy, WMD, leptomeningeal enhancement, or microhemorrhages). Brain abnormalities were asymmetrically predominant in the hemisphere ipsilateral to the hemifacial atrophy in 13 (81%) patients with epilepsy compared with 13 (20%) without epilepsy (P < .001). Two (12%) patients with epilepsy had brain abnormalities contralateral to hemifacial atrophy, and one (6%) had bilateral MR imaging brain abnormalities. Four (6%) and 14 (22%) patients without epilepsy had contralateral and bilateral brain MR imaging abnormalities, respectively.
As detailed in Table 2, the abnormalities with asymmetric involvement in the epilepsy group were hemispheric atrophy in 8 (50%) patients, WMD in 14 (87%), hemispheric microhemorrhage in 3 (18%, Fig 2), and leptomeningeal enhancement in 1 patient (6%, Fig 3). The abnormalities with asymmetric involvement in the nonepilepsy group were hemispheric atrophy in 3 (5%) patients, WMD in 15 (23%), hemispheric microhemorrhage in 3 (5%), and leptomeningeal enhancement in 1 (1%).
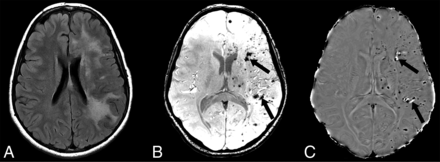
A, Axial FLAIR MR imaging in a patient with epilepsy shows severe, confluent left hemisphere white matter hyperintensity with extensive areas of punctate susceptibility artifacts throughout the left hemisphere on susceptibility-weighted imaging (B). Most of the hypointense foci on susceptibility-weighted imaging are hypointense on the matching phase image (C), consistent with prior microhemorrhages; however, few show hyperintense signal on the phase image (arrows in B and C), consistent with calcifications (confirmed by CT, not shown).
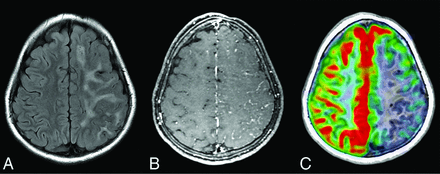
A, Axial FLAIR MR imaging in a patient with epilepsy shows severe, confluent left hemisphere white matter hyperintensity. B, Postcontrast T1-weighted MR imaging reveals leptomeningeal enhancement and sulcal effacement with decreased perfusion to the same area on arterial spin-labeling perfusion MR imaging (C).
Frequency and severity of unilateral brain abnormalities in patients with Parry-Romberg syndrome and epilepsy compared with cases without epilepsya
WMD was the most common finding in both groups. When we compared the degree of involvement between groups, the frequency and severity of WMD were higher in patients with epilepsy (P < .001) (Table 2). Hemispheric atrophy was also present with a higher frequency and severity in patients with epilepsy (P < .001). When present, hemispheric microhemorrhage was more severe in the epilepsy group (P = .015).
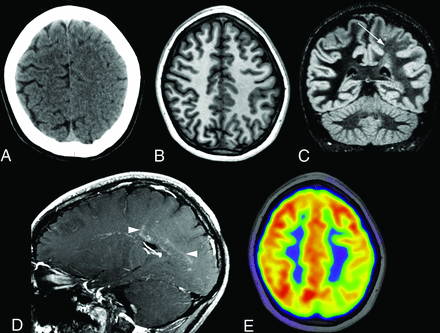
Additional intracranial findings in the entire cohort included bilateral frontal horn heterotopia (n = 1), bilateral supraclinoid aneurysms (n = 1), cortical and subcortical infarcts (n = 2), and subcortical dystrophic calcification (n = 2). Asymmetric sulcal effacement predominating in the frontoparietal region was found in 5 patients (6%, Figs 4 and 5), of whom, 3 had epilepsy. In 2 of the 3 patients, there was no change in sulcal effacement across multiple scans obtained for 17 months in 1 patient and 18 months in the second patient. Two patients with epilepsy had severe basal ganglia atrophy, located ipsilateral to the hemispheric atrophy and hemifacial atrophy. One patient per cohort was documented as having had progressive brain atrophy across the years. One patient with epilepsy and available FDG-PET showed hypometabolism in the area of sulcal effacement and WMD (Fig 4E). In another patient with epilepsy, arterial spin-labeling MR imaging showed hypoperfusion in the area of WMD and leptomeningeal enhancement (Fig 3C). 1H-MR spectroscopy (Fig 6) in a patient with epilepsy revealed a reduction in Cho:Cr ratio and a reduction in NAA in the area of WMD compared with the normal contralateral hemisphere.
A, Initial head CT in a patient with epilepsy reveals effacement of the sulci over the left parietal high convexity. B, Subsequent T1-weighted MR imaging performed 10 months later shows improvement in sulcal effacement, but with diffuse left hemisphere white matter hyperintensity on double inversion recovery MR imaging (C, arrow). D, Sagittal postcontrast T1-weighted MR imaging shows faint periventricular and perivascular enhancement (arrowheads). E, Concomitant FDG-PET shows left parietal hypometabolism in the affected area.
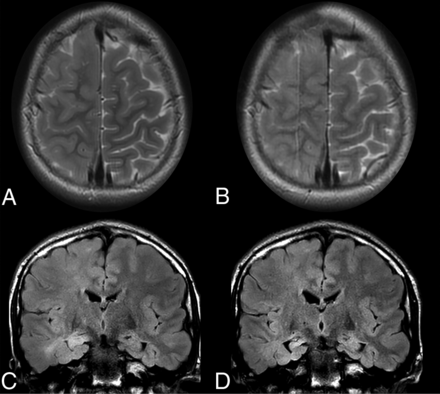
Spectrum of unilateral sulcal effacement. In 1 patient with epilepsy, axial T2-weighted MR imaging (A) shows right hemispheric sulcal effacement affecting the right frontal and parietal lobes, which remained unchanged on MR imaging performed 1 year later (B). C, Coronal FLAIR MR imaging in a second patient with epilepsy shows diffuse right hemisphere sulcal effacement with hyperintensity in the right hippocampal head, with resolution of sulcal effacement and development of right mesial temporal sclerosis on MR imaging approximately 6 months later (D).
Proton MRS in a 12-year-old boy with left-sided Parry-Romberg syndrome and epilepsy shows the spectrum from the normal-appearing right hemisphere white matter (A) compared with the area of white matter FLAIR hyperintensity in the left hemisphere (B). There is a relative reduction in Cho and NAA, with a slight elevation of Cr on the abnormal side. PPM indicates parts per million.
DISCUSSION
PRS is clinically suspected with facial asymmetry or hemiatrophy, often in association with neurologic, ophthalmologic, and orodental manifestations. The working diagnosis is made by clinically excluding mimickers such as localized scleroderma, Barraquer-Simons lipodystrophy, fat necrosis, contralateral hemihypertrophy, and congenital hemiatrophy. Rarely, laboratory testing or histopathology is performed to establish the diagnosis. Brain abnormalities are often seen in association with PRS, yet the frequency and severity have received limited attention. In this study, 57% of patients with a working or established clinical diagnosis of PRS and available MR imaging had at least 1 primary brain abnormality associated with PRS. The most common finding was WMD, which was observed asymmetrically in 29% of the cohort. Notably, unilateral-predominant brain abnormalities localized to the side with hemifacial atrophy were found in one-third of patients; due to the atrophic nature of the disorder, these are likely related to the pathophysiology of PRS.
Similar to our results, frequent imaging findings reported in other studies include ipsilateral white matter hyperintense signal on T2-weighted MR imaging corresponding to white matter hypoattenuation on CT, hemispheric brain atrophy, cavernous malformations/microhemorrhages, and subcortical calcifications predominantly noted in the frontal lobe.2,13,16⇓⇓⇓⇓-21 We found that the primary brain findings associated with PRS were more common in patients with epilepsy compared with those without epilepsy (94% versus 47%), as well as more commonly present on the side of craniofacial involvement in patients with epilepsy compared with those without epilepsy (81% versus 20%). When present, the degree of hemispheric atrophy, WMD, and microhemorrhage was more severe in those with epilepsy. In addition, 5 patients, including 2 without epilepsy, had remarkable asymmetric sulcal effacement located primarily in the frontoparietal region, a finding that has been rarely reported by other investigators.10,22⇓-24 Due to the low frequency of other findings, including bilateral frontal horn heterotopia, supraclinoid aneurysms, and small cortical and subcortical infarcts, it remains unclear whether these could be related to PRS.
It is often challenging to characterize the disease course, severity, and prognosis of PRS due to these overlapping features with ECDS or localized scleroderma.3 Currently, the most acceptable etiology associates PRS with an underlying progressive autoimmune neurovasculitis, though other theories have been proposed.11,21,25,26 Cerebral perivascular inflammation, leptomeningeal and cortical blood vessel endothelial degeneration, hyalinizing changes, and partial obliteration have been reported in PRS, possibly as a consequence of immune-mediated vascular injury and incomplete endothelial regeneration.27 These changes probably account for ischemia, hemorrhages, and breakdown of the blood-brain barrier. Robust response to steroids and immune-modulating treatment in slowing the disease process also supports this etiology, in addition to histologic and laboratory evidence of inflammation.1,2 Brain abnormalities in PRS have overlap with other conditions, such as Rasmussen encephalitis, an autoimmune disorder of childhood characterized by asymmetric neuroimaging findings, epilepsy, and hemiplegia.4 Characteristic craniofacial abnormalities in PRS may help differentiate the disorder from other entities.
Epilepsy can be a debilitating comorbidity in patients with PRS and further compromise quality of life, and it was found in 20% of patients in our series. A global, Internet-based survey of patients with PRS from 29 countries revealed that 11% had coexistent epilepsy,26 with the highest reported epilepsy incidence of 60.5% in a pooled literature review of patients with PRS.28 In a pediatric study of PRS with epilepsy, the median seizure onset was in the first decade.29 In our study, the age of seizure onset was often estimated; however, most patients had onset in childhood. In our cohort, the most common type of seizure was focal-to-bilateral tonic-clonic seizures, followed by focal with impaired awareness. Most cases (81%) of epilepsy had an abnormal EEG finding, with 50% demonstrating interictal epileptiform discharges. On the basis of our observations, the severity of brain abnormalities appears to be a risk factor for development of epilepsy. However, the widespread extent of the abnormalities limits the localizing ability of MR imaging in identifying the seizure-onset zone.30 Nevertheless, it appears that certain MR imaging features predispose to a higher likelihood of developing epilepsy in PRS. Similar to a recent study by Knights et al,31 our cohort showed a higher frequency and severity of ipsilateral WMD in epilepsy. Their study, however, failed to show a significantly greater frequency of hemorrhagic foci in epilepsy in contradiction to our findings. Last, abnormal MR imaging findings were more frequent in patients with epilepsy in their cohort, but this was not statistically significant.31 Both of these differences between studies may be related to the small number of subjects in their cohort.31
Currently, limited evidence exists regarding the role and findings of advanced brain imaging in PRS and epilepsy. Few patients in our cohort had such imaging performed, but findings were largely in agreement with prior reports.22,32 Hypoperfusion of the affected brain region has been shown in several prior studies using dynamic susceptibility contrast MR imaging or SPECT,19,22,33 which is in agreement with arterial spin-labeling results from 1 patient in our cohort. In 1 patient, a large region of hypometabolism was observed in the affected region on FDG-PET, which has also been previously reported.34 Last, few studies have shown the findings on 1H-MR spectroscopy in PRS. While findings have been mixed, most have shown a relative decrease in the Cho:Cr ratio and a decrease in NAA, similar to findings in 1 patient in our cohort.4,19,22,32
The descriptive findings in this study provide further insight into the need for brain imaging in patients with PRS and neurologic symptoms. Recognition of the frequency and spectrum of brain abnormalities in PRS is critical because the diagnosis was delayed for years and the opportunity for timely management was missed in many of our patients. Further longitudinal studies are needed to better understand the natural history and radiographic progression to diagnose and treat patients with PRS and epilepsy more optimally. Similarly, larger prospective studies are recommended to validate the prevalence of epilepsy and the response to therapy in patients with PRS.
There are several limitations to this study. The retrospective nature leads to selection bias, with heterogeneity and gaps in clinical documentation based on chart review. Nevertheless, the number of patients is relatively large and provides additional details of the association of epilepsy with PRS. The lack of established diagnostic criteria for PRS and frequent overlap with other entities such as ECDS could have led to challenges in establishing a definitive classification of progressive hemifacial atrophy. Referral to a tertiary care center likely overestimates the true incidence of a disease state and severity of associated abnormalities and, therefore, may not be generalizable to other populations.
CONCLUSIONS
In this study of 80 patients with a clinical diagnosis of PRS, brain abnormalities were frequently observed on MR imaging in the hemisphere ipsilateral to the hemifacial atrophy. Twenty percent of patients had focal epilepsies with most demonstrating motor signs and nearly 90% controlled by ASM. Lateralized brain abnormalities in the hemisphere ipsilateral to the hemifacial atrophy had a higher frequency and greater severity in patients with epilepsy compared with those without epilepsy. The most common MR imaging findings in both groups were WMD and hemispheric atrophy; both findings were more frequent and severe in patients with epilepsy.
Footnotes
C. De la Garza-Ramos and A. Jain contributed equally to this work.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received January 15, 2022.
- Accepted after revision March 21, 2022.
- © 2022 by American Journal of Neuroradiology