Abstract
SUMMARY: Nelarabine is a nucleoside analog critical for the treatment of patients with T-cell acute lymphoblastic leukemia/lymphoma. However, clinical peripheral and central neurologic adverse events associated with nelarabine administration have been reported. Neuroimaging of brain neurotoxicity has only been described in very few reports in pediatric patients so far. Six children with diagnosed T-cell acute lymphoblastic leukemia who clinically experienced possible, probable, or definite nelarabine-induced toxicity and underwent spine and/or brain MR imaging were reviewed. Neuroimaging findings showed a mixture of patterns including features of acute toxic leukoencephalopathy (seen in 6 cases), posterior reversible encephalopathy syndrome (2 cases), involvement of deep gray structures (1 case) and brainstem (2 cases), cranial and spinal neuropathy (2 cases each), and myelopathy (2 cases). Even though neuroimaging findings are nonspecific, the goal of this article was to alert the pediatric neuroradiologists, radiologists, and clinicians about the possibility of nelarabine-induced neurotoxicity and its broad neuroimaging spectrum.
ABBREVIATIONS:
- aBFM
- augmented Berlin-Frankfurt-Münster
- AE
- adverse event
- ATL
- acute toxic leukoencephalopathy
- PA
- post (after) nelarabine administration
- PRES
- posterior reversible encephalopathy syndrome
- T-ALL
- T-cell acute lymphoblastic leukemia
- T1+C
- contrast-enhanced T1WI
Nelarabine is a nucleoside analog and was approved by the US Food and Drug Administration for the treatment of patients with relapsed or refractory T-cell acute lymphoblastic leukemia (T-ALL) and T-cell lymphoblastic lymphoma in October 20051 and is now considered a gold standard therapeutic treatment option for de novo pediatric T-ALL.2 Clinical peripheral and central neurologic adverse events (AEs) associated with nelarabine administration have been described in both the pediatric and adult populations.3⇓⇓-6 The exact mechanism of nelarabine-associated neurotoxicity is still unclear and presumably multifactorial.7 There is only sparse literature addressing neuroimaging findings in nelarabine-induced neurotoxicity in children and adults.8⇓⇓⇓⇓⇓⇓⇓-16 To the best of our knowledge, only 9 case reports dealt with neuroimaging findings in nelarabine-associated neurotoxicity,8⇓⇓⇓⇓⇓⇓⇓-16 with all 9 reports depicting myelotoxicity, and so far, only 2 articles additionally showed intracranial toxicity in pediatric patients.11,13
Affected patients developed clinical signs and symptoms such as extremity weakness, reduction or loss of sensation, pain, ataxia, abnormal reflexes, and bladder or bowel dysfunction.8⇓⇓⇓⇓⇓⇓⇓⇓-17 Devastating and occasionally permanent CNS encephalopathy, often preceded by somnolence, while rare, has also been reported.3 However, neither imaging findings nor biomarkers of toxicity have been comprehensively described. On neuroimaging, an involvement of mainly the dorsal column of the spinal cord8⇓-10,12,14,16 and cross-sectionally extending myelopathy or involvement of the lateral column of the myelon have been reported.8,13 Imaging findings in nelarabine-associated intracranial toxicity have only been rarely described.11,13 Hence, the goal of this article was to describe the neuroimaging spectrum of nelarabine-induced neurotoxicity in a pediatric case series to make the pediatric neuroradiologists, radiologists and clinicians aware of the existence of nelarabine-induced neurotoxicity.
Case Series
Following institutional review board approval, a retrospective study was performed on the pediatric neuroradiology database and electronic health records of 2 quaternary care children’s hospitals (January 1, 2010, to September 30, 2021). Informed consent was waived for this purely retrospective study. Inclusion criteria were the following: 1) pediatric cases (< 18 years) with diagnosed T-ALL, 2) who received nelarabine, 3) experienced a neurotoxic event possibly, probably, or definitely nelarabine-related, and 4) had a spinal and/or brain MR imaging performed within 30 days of the clinical symptoms due to suspected nelarabine-induced toxicity. A board-certified pediatric oncologist (E.S.S.) and a board-certified pediatric neurologist (L.A.M.) reviewed the electronic health records and graded and assigned attribution to neurotoxic events on the basis of the current gold standard of AE reporting in the field of oncology:18 the National Cancer Institute Guidelines for Investigators: Adverse Events Reporting Requirements.19 Briefly, AEs were identified and graded (1, mild; 2, moderate; 3, severe; 4, life-threatening; 5, death) for severity using the Common Terminology Criteria for Adverse Events guide.20 Then attribution was assigned by the responsible physician using his or her best judgment based on factors including the subject’s baseline health status, disease history, comorbidities, and knowledge about the safety profile of the intervention (both personal and in reference to the interventions comprehensive AE and potential risks list19) in question.21
Defined AE categories were the following: “unrelated” (AE is clearly not related to the intervention), “unlikely” (AE is doubtfully intervention-related), “possible” (AE may be intervention-related), “probable” (AE is likely intervention-related), and “definite” (AE is clearly intervention-related).19 AEs listed as “possibly, probably, or definitely” related to the investigational agent or intervention are considered to have a suspected reasonable causal relationship to the investigational agent/intervention.19 The MR imaging studies were evaluated by 2 experienced board-certified pediatric neuroradiologists with >10 years and 10 years of experience, respectively (N.K.D. and J.N.W.). Each neuroradiologist performed the review independently at the respective institution. The reviewers, who were not blinded to the patient’s medical history, performed a descriptive review of the imaging data. In total 6 patients (6 boys; mean age, 9.67 years; age range, 6–14 years) were included.
Patient 1 was an 8-year-old boy with de novo T-ALL in induction failure who received a 5-day pulse of nelarabine before augmented Berlin-Frankfurt-Münster (aBFM)22 high-risk consolidation.5 In the evening post (after) nelarabine administration (PA), he developed an advancing depressed level of consciousness (grade 4, definitely nelarabine-related) during 48 hours, which began with somnolence and progressed to overt hallucinations, confusion, agitation without focus, and finally frank psychosis for which he was intubated and sedated. Initial brain MR imaging, 3 days PA demonstrated mild cerebral volume loss but was otherwise normal. Seizures and dystonia then became evident. Follow-up MR imaging, 12 days PA, demonstrated small patchy areas of T2-weighted/FLAIR hyperintensities in the cortical and subcortical regions of the bilateral posterior temporal and occipital lobes without corresponding diffusion restriction, paralleling posterior reversible encephalopathy syndrome (PRES). There was interval development of T2-weighted hyperintensities within the splenium of the corpus callosum, caudate head, and dorsal thalami, with progressive mild supratentorial volume loss and abnormal T2-weighted/FLAIR signal in the right pons with restricted diffusion.
Initial spine MR imaging showed mild enhancement of the cauda equina nerve roots and paraspinal soft-tissue edema. During this time, the patient became encephalopathic with progression to coma with minimal brainstem reflexes in addition to axonal neuropathy. Brain MR imaging after 30 days demonstrated interval development of multifocal T2-weighted prolongation in the subcortical WM now involving the bilateral frontal and parietal lobes and, to a lesser extent, the posterior centrum semiovale and periatrial WM, with improvement of cortical edema in the occipital and parietal lobes. Additional T2 hyperintensity was noted within the pons bilaterally and in the medulla oblongata, without a definitive matching diffusion restriction. Slight enhancement of cranial nerve II (cisternal portion) and avid enhancement of cranial nerves III and V were now apparent.
Forty-nine days PA there was progression with confluent nonenhancing T2-weighted/FLAIR hyperintensity throughout the entire supratentorial, subcortical, periventricular, and deep WM, corpus callosum, bilateral caudate head, dorsal thalami, anterior putamen, insula, pons, and medulla oblongata. Figure 1 summarizes the evolution of the MR imaging findings. Enhancement of cranial nerves II, III, and V remained present (Fig 2). Spinal MR imaging showed new abnormal T2 hyperintensity throughout the spinal cord, most prominent in the dorsal aspects (Fig 3), and equivocal enhancement of the nerve roots within the cauda equina as well as paraspinal soft-tissue edema. The CSF examination was negative for leukemic involvement. The symptoms secondary to the nelarabine-induced toxicity only minimally improved for months; without the ability to provide the required aggressive leukemia-directed chemotherapy secondary to his static encephalopathy, the patient eventually died of progressive leukemia.
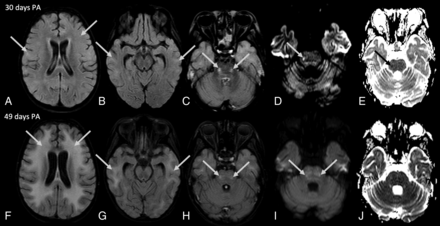
Brain MR images of an 8-year-old boy (patient 1) with definite nelarabine-induced toxicity. The CSF examination was negative for leukemic involvement. Axial FLAIR images with additional fat saturation (A–C and F–H), axial DWI (D and I), and corresponding ADC maps (E and J). Brain MR imaging performed 3 days PA demonstrated mild cerebral volume loss, but findings were otherwise normal (not shown). Follow-up brain MRIs 12 days PA (not shown), 30 days PA (A–E), and 49 days PA (F–J) demonstrate increasingly progressive T2-weighted/FLAIR hyperintensity, beginning as patchy areas in the cortical and subcortical regions (arrows in A and B) and expanding to confluent increased T2-weighted/FLAIR signal throughout the entire supratentorial subcortical, periventricular, and deep WM (arrows in F and G) as well as cortical regions. In addition, there was development of abnormal T2-weighted/FLAIR signal within the pons bilaterally (arrows in C and H) with transient corresponding diffusion restriction in the pons on the right side (arrows in D and E) and increased diffusion signal in the pons bilaterally (arrows in I) without definite decreased intensity on the corresponding ADC map on follow-up 49 days PA (J).
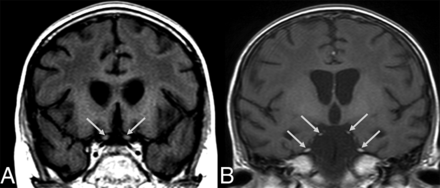
Brain MR images (49 days PA) of an 8-year-old boy (patient 1) with definite nelarabine-induced toxicity. The CSF examination was negative for leukemic involvement. Coronal T1+C (A and B) demonstrates slight enhancement of cranial nerve II (cisternal portion, arrows in A) and avid enhancement of cranial nerves III and V (arrows in B). In addition, the confluent leukoencephalopathy and a slight cerebral volume loss with progressing ex vacuo widening of the ventricles are evident.
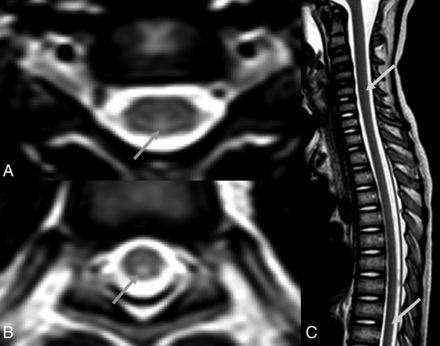
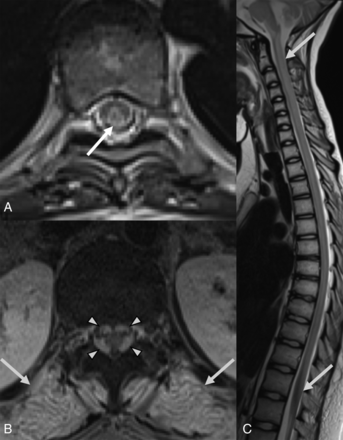
Spinal MR images (49 days PA) of an 8-year-old boy (patient 1) with definite nelarabine-induced toxicity. The CSF examination was negative for leukemic involvement. Axial T2-weighted images (A and B) at the level of C5 and T10, respectively, and sagittal T2-weighted image (C). Images show abnormal T2 hyperintensities throughout the spinal cord, most prominent in the dorsal aspects (arrows in A, B, and C).
Patient 2 was a 14-year-old boy diagnosed with early T-cell precursor ALL. Initiation of the best available therapy chemotherapy with dexamethasone, vincristine, and daunorubicin followed. He received a 5-day pulse of nelarabine as salvage chemotherapy. PA, he developed seizures and progressive severe encephalopathy (grade 4, definitely nelarabine-related). Beginning at 9 days PA, neuroimaging demonstrated features of PRES, which resolved spontaneously by 20 days PA. On the day 20 examination and during the subsequent 5 months (6 additional MRIs), the patient developed progressive leukoencephalopathy with increased T2-weighted signal and partially transient diffusion restriction starting in the WM of the bilateral occipital lobes. There was subsequent involvement of the WM of the pre- and postcentral gyri and posterior corpus callosum, progressing to diffuse WM involvement (Fig 4). In addition, T2 hyperintensity was seen in the inferior cerebellar peduncles and along several WM tracts (including the corticospinal tract and medial longitudinal fasciculus). On contrast-enhanced T1WI (T1+C), enhancement of cranial nerves III, V, VI, VII, VIII, IX, and XII was noted (Fig 5). Progressive diffuse cerebral volume loss was evident. In addition, during the course of 5 months, 6 spinal MRIs were performed, showing progressive development of denervation edema and enhancement of the paraspinal muscles, increasing nerve root enhancement, and gradual development of T2 hyperintensity of the dorsal column, corticospinal tract, and spinothalamic tract (Fig 6). The CSF examination was negative for leukemic involvement. The patient died secondary to end-stage recurrent leukemia that was complicated by severe chemotherapy-induced encephalopathy.
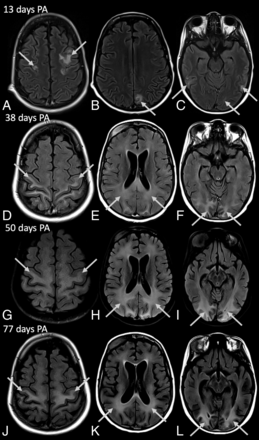
Brain MR images of a 14-year-old boy (patient 2) with definite nelarabine-induced toxicity. The CSF examination was negative for leukemic involvement. Axial FLAIR images (A–F and J–L) and axial FLAIR with additional fat saturation (G–I). During 5 months (exemplary MR images at 13, 38, 50, and 77 days PA are shown), the patient first demonstrated features of PRES in the occipital, parietal, frontal, and temporal lobes (arrows in A–C), followed by progressive leukoencephalopathy with increased T2-weighted/FLAIR signal starting occipitally and spreading across the pre- and postcentral gyrus and parietal and frontal WM to diffusely affect the WM (arrows in D–L). In addition, progressive global atrophy is clearly evident.
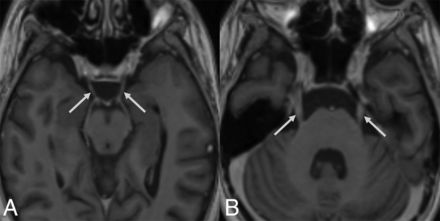
Brain MR images (13 days PA) of a 14-year-old boy (patient 2) with definite nelarabine-induced toxicity. The CSF examination was negative for leukemic involvement. Axial T1+C (A and B) demonstrates exemplary enhancement of the cranial nerves III (arrows in A) and V (arrows in B).
Spinal MR images (38 days PA) of a 14-year-old boy (patient 2) with definite nelarabine-induced toxicity. CSF examination was negative for leukemic involvement. Axial T2-weighted image (A) shows abnormal signal in the bilateral posterior columns (arrow). Axial T1+C (B) at the level of the conus medullaris demonstrates diffuse enhancement of the ventral and dorsal nerve roots of the cauda equina (arrowheads). In addition, there is diffuse enhancement of the paraspinal musculature reflecting acute denervation edema (arrows in B). Sagittal T2-weighted image (C) demonstrates longitudinally extensive dorsal column involvement extending from the cervicomedullary junction through the conus (arrows).
Patient 3 was a 10-year-old boy with an isolated CNS relapse of T-ALL. He received 2 doses of high-dose methotrexate, followed by the nelarabine, cyclophosphamide, and etoposide regimen.23 Twenty-one days PA, the patient presented to the emergency department with somnolence (grade 3, probably nelarabine-related), nausea, and vomiting. The only confounding chemotherapy was intrathecal triples (methotrexate, cytarabine, hydrocortisone). Brain MR imaging showed increased T2-weighted/FLAIR signal in the periventricular and deep WM (predominantly frontoparietal) and smaller areas in the left temporal subcortical WM, without corresponding diffusion restriction. Two follow-up MRIs 139 and 154 days PA demonstrated moderate progression of periventricular and deep WM elevated signal, which was now seen in all lobes, suggestive of acute toxic leukoencephalopathy (ATL) (Fig 7). There was no corresponding diffusion restriction. Progressive cerebral volume loss was evident. At MR imaging, the CSF was negative for leukemic involvement. Nelarabine-induced toxicity fully resolved, but the patient eventually died of graft failure after undergoing a hematopoietic stem cell transplant.
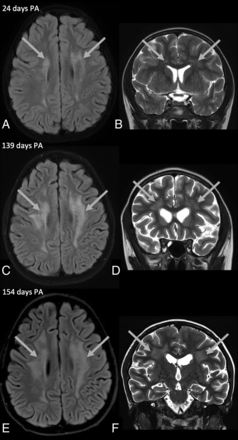
Brain MR imaging obtained in a 10-year-old boy (patient 3) with probable nelarabine-induced toxicity. The CSF examination was negative for leukemic involvement. Axial FLAIR with additional fat-saturation (A, C, and E) and coronal T2-weighted (B, D, and F) images obtained 24, 139, and 154 days PA. Images (A and B) show increased T2-weighted/FLAIR signal in the periventricular and deep WM, predominantly frontoparietal (arrows in A and B). Two follow-up MRIs (C and D and E and F) demonstrate progressive periventricular and deep WM elevated signal (arrows in C, D, E, and F).
Patient 4, a 10-year-old boy, was diagnosed with de novo T-ALL and CNS involvement and received an intended 5-day pulse of nelarabine followed by aBFM high-risk consolidation.5 On day 2 of nelarabine administration, he presented to the emergency department feeling dizzy and falling. Symptoms progressed to hypersomnia (grade 3, probably nelarabine-related), confusion, and short-term memory loss (encephalopathy grade 4, probably nelarabine-related). Brain MR imaging demonstrated subtle increased T2-weighted/FLAIR signal in the WM adjacent to the trigones of the lateral ventricles bilaterally. At MR imaging, the CSF examination was negative for leukemic involvement. The last nelarabine administration was stopped and symptoms slowly resolved and he remains in complete leukemic remission.
Patient 5 was a 10-year-old boy with de novo T-ALL and CNS involvement. He received an intended 5-day pulse of nelarabine followed by an aBFM high-risk consolidation.5 On day 3, the patient noted right upper and lower extremity weakness, prompting nelarabine discontinuation. On neurologic consultation, a distal ascending peripheral neuropathy was diagnosed (grade 3, probably nelarabine-related). Spinal MR imaging findings were unremarkable. Brain MR imaging was additionally performed and revealed subtle T2-weighted/FLAIR hyperintensities in the frontal bilateral centrum semiovale with no corresponding diffusion restriction. At the time of the MR imaging, the CSF examination was negative for leukemic involvement. The patient’s nelarabine toxicity fully resolved, and he remains in complete leukemic remission.
Patient 6, a 6-year-old boy, was diagnosed with an isolated CNS relapse of T-ALL after experiencing visual disturbance, headaches, and emesis. Neurologic examination noted minimal facial movements, and an ophthalmologic examination revealed considerable disease involvement of both retinas. He received a 5-day pulse of nelarabine, followed by an aBFM high-risk consolidation.5 Thereafter, he received 2 additional 5-day nelarabine monotherapy courses every 28 days. Ten days PA, the patient presented to the emergency department with encephalopathy and hypersomnia (grade 3, possibly nelarabine-related). His brain MR imaging revealed increased T2-weighted signal in the periventricular and deep WM without corresponding diffusion restriction. Follow-up MR imaging 25 days PA demonstrated progression, now including T2-weighted signal abnormalities of the brainstem and central GM. In addition, the MR imaging studies demonstrated bilateral retinal leukemic involvement and presumed parenchymal, leptomeningeal, and pituitary stalk leukemic involvement. The patient’s toxicity resolved, but he died shortly thereafter secondary to progressive leukemia.
DISCUSSION
In this case series, we present 6 male pediatric patients with definite (2 cases), probable (3 cases), and possible (1 case) nelarabine-induced neurotoxicity. AEs were acute (during/shortly after) or early delayed (weeks) after nelarabine treatment, and patients presented with signs and symptoms of peripheral and central neurotoxicity. Our cohort showed a mixture of neurotoxic patterns, including imaging features of ATL (seen in 6 cases), PRES (2 cases), involvement of deep GM structures (1 case) and brainstem (2 cases), cranial and spinal neuropathy (2 cases each), and myelopathy (2 cases).
Neurotoxicity can be seen in a wide variety of therapies, including immunosuppressive, chemotherapeutic, antibiotic, and anti-epileptic agents.24⇓⇓-27 There are known common primary medication-related neurotoxic injury patterns on MR imaging and several agents that follow these pathognomonic patterns, while other agents lead to mixed or more enigmatic patterns.24,25,28
A common well-defined typical imaging pattern of neurotoxicity is ATL, and MR imaging findings typically demonstrate nonenhancing diffuse symmetric T2-weighted/FLAIR hyperintensities within the deep WM across multiple vascular beds, often associated with restricted diffusion.24⇓-26,28 The ATL pattern can be seen with a wide spectrum of different agents, including, among others, methotrexate, 5-fluorouracil, and fludarabine.28,29 Presenting symptoms include seizures, encephalopathy, cognitive dysfunctions, and visual impairment.28,29 ATL is thought to be caused by microvasculature damage or an excitotoxic effect on the brain.28,30
PRES is another well-known injury pattern to the pediatric brain. PRES is a clinicoradiologic syndrome, clinically presenting with headache, seizures, altered mental status, and visual impairments. On MR imaging, PRES is typically characterized by bilateral vasogenic edema seen as T2-weighted/FLAIR high signal mainly in the posterior cerebral cortex and subcortical WM, followed by involvement of the frontal and temporal lobes.24,25,28⇓-30 The brainstem, basal ganglia, or cerebellum is less commonly affected.28,29 Atypical imaging findings include leptomeningeal, cortical, or, rarely, nodular enhancement, hemorrhage, and restricted diffusion.28,29 Even though PRES is usually reversible on discontinuation of the toxic agent, there are cases with irreversible brain damage.28⇓-30 Hypertension, induction chemotherapy, and treatment with immunosuppressive agents such as cyclosporine, tacrolimus, and corticosteroids have been described as risk factors for PRES.29 Normal blood pressure can be found in cases of PRES, especially in the context of chemotherapy, immunosuppressive therapy, and sepsis.29 The exact pathophysiology is still not fully understood. PRES is thought to be due to a failure of cerebrovascular autoregulation with disruption and leakage of the blood-brain barrier.24,25,27,28 The paucity of sympathetic innervation in the vertebrobasilar territory is postulated to be the reason for the posterior predilection.25,28
Less common patterns include the involvement of the deep brain structures such as the thalami, basal ganglia, anterior commissure, dentate nuclei, brainstem, and corpus callosum and have already been described with, for example, metronidazole or vigabatrin and result in T2-weighted/FLAIR increased signal and occasionally restricted diffusion.25 Myelopathy can result from different agents, including chemotherapeutics.24 In the acute phase, MR imaging findings may be normal, followed by focal cord swelling with T2-weighted/FLAIR hyperintensities and, potentially, intramedullary enhancement.24 Peripheral and specific cranial neuropathies are common neurologic complications of cancer treatment, and if MR imaging is performed, they may be seen as contrast enhancement of the nerve root or cranial nerves, respectively.31
DWI plays an important role because lesions in ATL or PRES may be more conspicuous on DWI than on conventional T2-weighted/FLAIR images. Although restricted diffusion (cytotoxic edema) is typically evident in ATL and can be an atypical imaging feature of PRES, there is mostly clinical improvement, and in contrast to ischemic-induced cytotoxic edema, a resolution of the diffusion restriction may be seen (Fig 1).25,28,29
Recognizing the involvement or sparing of the subcortical U-fibers can be helpful in determining the etiology and extent of the disease process.32 Subcortical U-fibers, reflecting short association fibers, comprise thin bundles of myelinated nerve fibers that connect the cerebral cortices of adjacent gyri.32 While in the ATL pattern, seen with different agents including methotrexate, the subcortical U-fibers are mostly spared, in PRES they are frequently involved.32 In our cohort, 2 patients had a mixture of neuroimaging findings, including features of PRES and ATL, resulting in both partial sparing and partial involvement of the subcortical U-fibers (Figs 1 and 4). In 4 patients, the neuroimaging findings paralleled the ATL pattern, leading to a sparing of the U-fibers (Fig 7).
Leptomeningeal, cortical, or nodular contrast enhancement may be the result of neurotoxic-induced disruption of the blood-brain and blood-nerve barriers.26,31 In our cohort, we found enhancement of several cranial nerves on T1+C (patients 1 and 2), a finding seen in a wide variety of entities including neurotoxicity.33
A systematic, larger series review of the spectrum of neuroimaging findings seen in nelarabine-induced neurotoxicity of the brain is still lacking. Hartz et al13 reported T2-hyperintense lesions in the superior cerebellar peduncles, cerebral peduncles, and pons and within several cranial nerves; postmortem histopathologic samples revealed further damage in the basal ganglia, thalamus, mammillary body, and occipital WM. Ewins et al11 found leukoencephalopathy with T2-weighted/FLAIR signal increase in the subcortical and deep cerebral WM. Similar to Hartz et al, they further reported T2-weighted/FLAIR hyperintensities in the pons, middle cerebellar peduncles, and posterolateral aspect of the medulla oblongata.11
Neurotoxicity is, after myelosuppression, the second most dose-limiting factor of cancer treatment.31,34 It is thought to occur by direct damage to neurons and/or glia or indirectly by modifying the surrounding microenvironment, leading, for example, to localized vascular injury and resulting in a disruption of the blood-brain, blood-CSF, and blood-nerve barriers.26,31
All patients in our case series were boys. Interestingly, we found more case reports describing neurotoxicity in males9,11,13⇓⇓-16 than in females.8,10,12 The significance of this finding is as yet undetermined. In general, the occurrence of nervous system toxicity is based on a broad number of different factors including drug dosage, route of administration, potential interactions with other administered agents or pre-existing structural nervous system diseases, and the individual patient vulnerability such as, for example, polymorphisms in genes related to neurogenesis.9,31
There is no strict correlation between clinical findings of neurotoxicity and imaging.35 Our case series comprised 1 patient who had only symptoms of peripheral neuropathy but also demonstrated mild leukoencephalopathy on brain MR imaging.
We are aware of several limitations in our study. First, this case series is based on a retrospective study design with a very limited number of pediatric patients and with MR imaging performed on various scanners. Second, because nelarabine is administered as a part of oncologic treatment regimens, correlated imaging findings cannot be attributed to nelarabine-induced toxicity with absolute certainty. Third, due to the retrospective design, we are aware of the lack of statistical data resulting from a prospective inclusion and subsequent MR imaging of all patients treated with nelarabine during the survey period and resulting from a correlation of imaging findings with the dosage or interval of nelarabine administration as well as clinical signs/symptoms. Fourth, no postmortem histologic samples were obtainable for correlation. Finally, no long-term follow-up was available, limiting our analysis on the long-term reversibility of imaging findings as well as limiting the examination of a possible late impact on brain cognition. Subsequent future prospective studies with larger cohorts, standardized imaging protocols, and longitudinal follow-up are needed to answer these questions.
CONCLUSIONS
With this case series, we present 6 pediatric cases of possible, probable, or definite nelarabine-induced neurotoxicity. Patients presented with acute or early-delayed AE after nelarabine treatment with signs/symptoms of peripheral and central neurotoxicity. We found a wide spectrum of neuroimaging findings, including features of PRES, ATL, involvement of deep gray structures and brainstem, cranial and spinal neuropathy, and myelopathy. Even though the mentioned neuroimaging findings are completely nonspecific and cannot be attributed to nelarabine-induced toxicity with absolute certainty, the goal of this case series was to alert the pediatric neuroradiologists, radiologists, and clinicians about the possibility of nelarabine-induced neurotoxicity and its resulting broad neuroimaging spectrum. The awareness of nelarabine-induced neurotoxicity could assist the monitoring of therapeutic interventions because dosage adjustment or agent discontinuation may prevent further neurologic injury.
Footnotes
B.L. Serrallach was supported by the Edward B. Singleton endowment.
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received July 29, 2022.
- Accepted after revision September 29, 2022.
- © 2022 by American Journal of Neuroradiology