Abstract
BACKGROUND AND PURPOSE: Reduced olfactory function is the symptom with the highest prevalence in coronavirus disease 2019 (COVID-19) with nearly 70% of infected individuals experiencing partial or total loss of their sense of smell at some point during the disease. The exact cause is not known, but beyond peripheral damage, studies have demonstrated insults to both the olfactory bulb and central olfactory brain areas. However, these studies often lack both baseline pre-COVID-19 assessments and control groups, and the effects could, therefore, simply reflect pre-existing risk factors.
MATERIALS AND METHODS: Shortly before the COVID-19 outbreak, we completed an olfactory-focused study, which included structural MR brain images and a full clinical olfactory test. Opportunistically, we invited participants back 1 year later, including 9 participants who had experienced mild-to-moderate COVID-19 (C19+) and 12 who had not (C19−), creating a natural pre-post experiment with a control group.
RESULTS: Despite C19+ participants reporting subjective olfactory dysfunction, few showed signs of objectively altered function. Critically, all except 1 individual in the C19+ group had reduced olfactory bulb volume (average reduction, 14.3%), but this did not amount to a significant statistical difference compared with the control group (2.3%) using inference statistics. We found no morphologic differences in olfactory brain areas but stronger functional connectivity between olfactory brain areas in the C19+ group at the postmeasure.
CONCLUSIONS: Our data suggest that COVID-19 might cause long-term reduction in olfactory bulb volume and altered functional connectivity but with no discernible morphologic differences in cerebral olfactory regions.
ABBREVIATIONS:
- C19+
- positive for COVID-19
- C19−
- negative for COVID-19
- COVID-19
- coronavirus disease 2019
- OB
- olfactory bulb
- SARS-CoV-2
- Severe Acute Respiratory Syndrome coronavirus 2
Olfactory dysfunction is a key symptom of coronavirus disease 2019 (COVID-19).1 The reported prevalence of complete olfactory loss (anosmia) is about 50%, with an additional 10%–20% reporting less severe olfactory dysfunction at some point during the disease.2,3 Thus, a reported reduced sense of smell is the symptom with the highest odds ratio in nonhospitalized cases.4,5 Despite the clear clinical link between olfactory dysfunction and COVID-19, our understanding of the mediating mechanisms is limited.
Much like the Severe Acute Respiratory Syndrome coronavirus 1 and influenza viruses,6 Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2) can invade the central nervous system through the olfactory mucosa via a retrograde route.7 SARS-CoV-2 nucleoproteins and associated inflammation have been detected in infected animal models along the entire olfactory route from the olfactory sensory neurons to the olfactory bulb (OB).7 In humans, there is indirect and mixed evidence of SARS-CoV-2 as a neurotropic virus. Studies have demonstrated postmortem brain pathologies after COVID-19 but without clear evidence of SARS-CoV-2 RNA presence.8 There is further conflicting or weak evidence of neuroinvasion within the olfactory system in humans with a dominance of case studies or assessment of severe cases. A postmortem case study found low levels of virus RNA in the OB,9 and several neuroimaging studies have demonstrated anosmia-related edemas and abnormalities evident on CT or MR images of the OB post-COVID-19 infection.10⇓⇓⇓-14 On the other hand, the absence of a significant difference in OB volume between a COVID-19–related anosmia group and a general postviral anosmia group has been reported,15 and a postmortem tissue examination from patients with severe COVID-19 indeed found virus in the olfactory nerve but only in the leptomeninges layer of the OB,16 leaving no consensus as to whether COVID-19 is a neurotropic virus.
Full psychometric assessment of olfactory function and measures of morphology of the central olfactory system are needed to understand the central mechanisms of COVID-19-related olfactory dysfunction in humans, preferably from the same individual both before and after infection and with the inclusion of a relevant control group for comparison. Nevertheless, inducing COVID-19 for experiments is ethically questionable. However, in the months leading up to the first COVID-19 outbreak in Stockholm (late 2019 to early 2020), we acquired full-scale psychometric olfactory assessments and structural MR images from a group of healthy individuals as a control group in a study assessing the neural effects of olfactory dysfunction. One year into the pandemic and before the general vaccination program was initiated in Sweden, we recruited participants who had COVID-19 since the first study and participants who had not. In this natural experiment with pre- and postmeasures in both COVID-19-affected individuals and a comparable control group, we aimed to determine whether COVID-19 alters olfactory function and the morphology of cerebral areas associated with olfactory processing: the olfactory bulb, anterior and posterior piriform cortex, and central areas of the orbitofrontal cortex. Second, we aimed to assess potential links between morphologic changes and changes in olfactory functions due to COVID-19. In addition, we measured functional connectivity between olfactory areas in both groups in the poststudy. Critically, we preregistered our hypotheses and analyses before assessing the data. A previous version of this article exists as a preprint.17 Results and main conclusions are similar between this and the preprint versions, but textual differences exist.
MATERIALS AND METHODS
All methods and analyses are according to our preregistration (https://aspredicted.org/wr4d9.pdf) unless otherwise explicitly stated.
Participants
All participants were recruited from a previous study, referred to here as the “prestudy” (n = 52) that took place September 2019 to February 2020. A total of 40 individuals (77%) responded to our request to participate in the poststudy, of which we classified 9 (6 women) as having been infected with COVID-19 (C19+) with mild-to-moderate symptoms. Eight of these reported having tested positive for either ongoing infection (n = 4) and/or antibodies (n = 5), and 1 reported being diagnosed by a medical doctor without being tested (this happened before testing without hospital admittance was available in Sweden). We also recruited 12 control participants (5 women) classified as not having contracted COVID-19 (C19−) on the basis of the absence of symptoms during the pandemic as well as antibody tests negative for COVID-19 (n = 10). One individual in the negative for COVID-19 (C19−) group tested positive for ongoing infection a few weeks after the poststudy. Once recovered, she participated in the poststudy once more; this time as a C19+ participant, thereby contributing to both the C19− and C19+ groups. The C19+ participants were, on average, 38 (SD, 8) years of age (range 30–51 years), and the C19− participants were, on average, 33 (SD, 7) years of age (range, 26–49 years) at the prestudy. The poststudy took place between 3 weeks and 12 months after infection for the C19+ group (mean, 7 [SD, 4] months). In the prestudy, all participants reported having normal or corrected-to-normal vision, hearing, and olfactory function.
All procedures were approved by the Swedish Ethical Review Authority, and all participants provided written informed consent before participating in both the pre- and the poststudy.
Psychometric Odor Assessment
Individual olfactory performance was assessed after the MR imaging data acquisition in both the pre- and poststudy. We measured the odor-detection threshold, olfactory quality discrimination, and cued olfactory identification (sum score, threshold-discrimination-identification) using the validated Sniffin’ Sticks smell tests (Burghart).
Neuroimaging Acquisition and Processing
MR Imaging Data Acquisition.
For both sessions, the same 3T Magnetom Prisma MR imaging (Siemens) scanner with a 20-channel head coil was used. We acquired structural images in both studies using identical protocols with a 3D MPRAGE T1-weighted sequence (208 slices, TR = 2300 ms, TE = 2.89 ms, flip angle = 9°, voxel size = 1 × 1 × 1 mm, FOV = 256 × 256 voxels). To assess potential effects of COVID-19 on olfactory functional connectivity, the poststudy included a 12-minute functional resting-state scan using an echo-planar imaging sequence (56 slices, TR = 1700 ms, TE = 30 ms, flip angle = 70°, voxel size = 2.2 × 2.2 × 2.2 mm, FOV = 94 × 94 voxels). For 1 participant in the C19+ group, all neuroimaging data were excluded from analysis due to excessive motion artifacts, making delineation of the olfactory bulbs problematic.
Volumetric Measures.
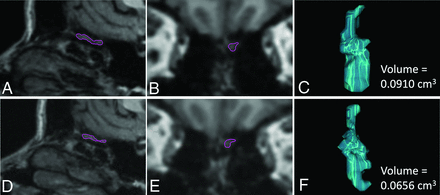
OB volume was assessed manually for each structural image and hemisphere (Fig 1). Data from both sessions for each participant were assigned to 1 of 2 experienced neuroradiologist raters (coauthors V.L. and M.B.) who were naïve to whether participants belonged to the C19+ or C19− groups. For the full cerebral cortex, voxel-based morphometry analysis was performed using the longitudinal pipeline in the Computational Anatomy Toolbox, Version 12.8 for SPM12 (http://www.fil.ion.ucl.ac.uk/spm/software/spm12). Our preregistered analysis plan was based on a cross-sectional publication,18 whereas the current study has a longitudinal nature. Consequently, we used the longitudinal pipeline in the CAT12 toolbox,19 which entails additional intrasubject processing steps and the use of Geodesic Shooting20 instead of the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra Toolbox (DARTEL, part of SPM) for spatial registration.
Illustration of the volumetric measurements of the left OB in a C19+ participant, in whom a decrease in OB volumes was observed 11 months after infection compared with the premeasure. The upper panel shows sagittal (A) and coronal (B) reconstructions of the high-resolution 3D T1WI sequence from the premeasure MR imaging with the left OB delineated in purple and the resulting computed OB volume (C). The corresponding data are shown for the postmeasure in the lower panel (D–F).
Connectivity Measures.
Data preprocessing and denoising of functional images was performed using SPM12 and the CONN functional connectivity toolbox, Version 20.b (https://web.conn-toolbox.org/resources/documentation) following the steps outlined in Peter et al.21 No group differences in motion were demonstrated on the basis of Welch t tests of mean framewise displacement (C19+: 0.2 mm; C19−: 0.18 mm; t(16.4) = 1.25, P = .31) or the number of volumes with a framewise displacement >0.5 mm (C19+: 6.1; C19−: 4.9; t(13.7) = 0.39, P = .7).
Creation of ROIs.
Three ROIs were included to assess potential COVID-19-related alterations in cortical structure and functional connectivity in areas associated with olfactory processing: the anterior piriform cortex, posterior piriform cortex, and orbitofrontal cortex, all based on a published olfactory activation likelihood analysis22 and restricted to core processing areas.23 Auditory and visual ROIs corresponding to the functions of the olfactory ROIs were included as control regions for the functional connectivity analysis: the primary auditory cortex, higher order auditory cortex, primary visual cortex, and the lateral occipital complex; all defined in Porada et al.23
Statistical Analyses
Change scores were calculated as (post) – (pre) for each individual for the measures that were acquired in both sessions.
Olfactory Function.
The hypothesis of reduced olfactory function (more negative change score) in the C19+ group compared with the C19−group was assessed with a 1-sided Welch t test, α = .05, for all tests unless otherwise stated, on the olfactory threshold-discrimination-identification change scores.
Volumetric Measures.
We computed the average OB volume across the left and right hemispheres. Our hypothesis of reduced OB volume in the C19+ compared with the C19− group was assessed using a 1-sided Welch t test on the change scores. In addition, we performed a nonpreregistered binomial test to assess whether the number of participants with an increased or decreased OB volume differed between groups. In this test, each participant was classified as having an either increased or decreased OB volume (positive or negative change score, respectively), and the number of participants in each category in each group was then compared with the expected null distribution.
GM volume in the preregistered olfactory ROIs (anterior piriform cortex and posterior piriform cortex) as well as in an additional olfactory ROI (orbitofrontal cortex) was extracted and then averaged over the hemispheres. Our hypothesis of reduced GM volume in the C19+ group compared with the C19− group was tested using 1-sided Welch t tests on the change scores. We also performed an exploratory whole-brain-group comparison of voxelwise tests of the interaction between the factors group (C19+/C19−) and time (pre/post), with a threshold of P < .001 and a minimum cluster size of 10 voxels.
Connectivity Measures.
Blood oxygen level–dependent time-series were extracted from all ROIs, and functional connectivity between the regions was calculated on the basis of a pair-wise Pearson correlation within the 3 sensory systems separately. The correlation values were Fisher z-transformed for statistical comparisons between the 2 groups, performed using 2-sided Welch t tests.
Data Availability
Data not provided in the article because of space limitations may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results.
RESULTS
We first assessed the objective change in olfactory function due to COVID-19 by comparing the change in threshold and threshold-discrimination-identification scores of the C19+ and C19− groups. We hypothesized that the C19+ group would demonstrate a larger reduction in olfactory performance than the control group. However, there was no significant difference between the groups in either threshold (t (12.1) = 0.97, P = .82) or threshold-discrimination-identification (t (9.4) = 0.60, P = .72; 1-sided Welch’s t tests). Contrary to this lack of an apparent objective difference between the C19 + and C19− groups in olfactory function, 4 of the 9 participants in the C19+ group did experience subjective olfactory dysfunction at the time of the poststudy, including 1 case of parosmia and 1 case of potential phantosmia (Table). The self-estimated overall olfactory function, compared with the function around the time of the prestudy, ranged from 50% to 100% (mean, 87.5%, SD, 17.9%) in the C19+ group, whereas for the C19− group, 100% of participants rated themselves as experiencing no difference in olfactory performance.
Self-reported acute and persisting chemosensory-related symptoms for all C19+ participants and demographic information. Rows indicate participants
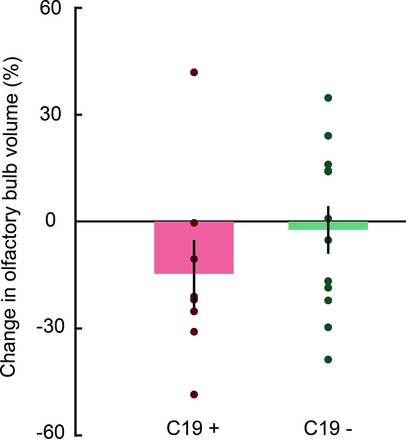
Next, we determined whether COVID-19 might lead to a long-term reduction in OB volume. On average, the OB volume in the C19+ group was reduced by 14.6% (SD, 26.8%) in the post-COVID measure compared with the pre-COVID measure. In the C19− group, the corresponding number was a 2.3% (SD, 23.2%) volume reduction (Fig 2). Our preregistered 1-sided Welch t test on the change scores showed that this difference between groups was nonsignificant according to our α criterion, t(15.2) = 1.3, P = .1, Hedges’ g = .58. However, in the C19+ group, 87.5% of participants (7 of 8) demonstrated a reduction in OB volume (mean for these participants, 22.7% [SD, 15.2%]). When assessing the likelihood that the observed reduction in OB volume in the C19+ occurred due to chance (binomial test), we found that the probability that ≥7 C19+ participants would demonstrate a reduction was P = .035, z = 1.76.
Mean (bars) and individual (dots) OB volume change from the pre- to the poststudy for the C19 + and C19− groups. Error bars denote ±1 standard error of the mean.
Although we did not systematically interview participants about parosmia and phantosmia symptoms nor did we test them for such, we made an inventory of their spontaneous reports of acute remaining symptoms, which were both of a chemosensory and general nature. The details of the self-reported acute and remaining chemosensory symptoms and demographics for each participant are shown in the Table. The participant who contributed to both the C19− and C19+ groups had a relatively unchanged OB volume when participating in the C19− group (0.8% increase) but showed a 31% volume reduction shortly after COVID-19 infection. Her persistent olfactory problems included a constant strange stale taste in the mouth, a potential sign of phantosmia, as well as selective anosmia for 3 of the sample odors in the discrimination task; something that she did not recall experiencing at the premeasure. She also reported experiencing selective anosmia in her everyday life. The C19+ participant who showed an increase in OB volume (outlier in Fig 2) also reported persistent chemosensory problems, including signs of parosmia. These were the only 2 participants who reported signs of parosmia or phantosmia. One participant reported sensing more bitterness, and another reported poor taste, especially on the tip of the tongue, both of which might be signs of actual gustation problems.
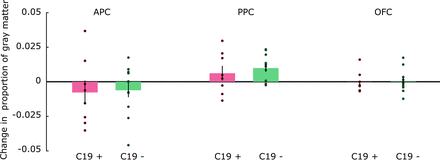
Next, we assessed whether COVID-19 leads to loss of GM volume in central olfactory areas by determining whether the GM volume change score was different in the C19+ group compared with the C19− group. We did not find any statistically significant differences in the 3 ROIs (all, Ps > .27; Fig 3). Finally, in an exploratory analysis, we assessed whether there were signs of volumetric changes to any nonhypothesized brain areas by performing a whole-brain contrast between C19+ and C19− groups. No voxels survived the set statistical threshold.
Mean (bars) and individual (dots) pre- to postchange scores for GM volume within the C19 + and C19– groups, separately for each ROI. Error bars denote ±1 standard error of the mean. APC indicates anterior piriform cortex; PPC, posterior piriform cortex; OFC, orbitofrontal cortex.
Finally, we assessed whether COVID-19 could be linked to alterations in resting-state functional connectivity between core olfactory regions. The C19+ group demonstrated an increase in functional connectivity between the orbitofrontal cortex and the anterior piriform cortex, t(14.6) = 3.92, P < .005, Hedges’ g = 1.73, as well as the orbitofrontal cortex and the posterior piriform cortex, t(12.1) = 3.07, P < .01, Hedges’ g = 1.42 compared with the C19− group. However, we found no significant group differences between the closely located anterior and posterior piriform cortex, t(13.6) = 0.15, P = .89, Hedges’ g = .07. Likewise, we found no significant group differences between auditory and visual control regions (all, Ps > .17).
DISCUSSION
In the present study, we used a unique group of participants that allowed us to assess COVID-19-dependent effects on the morphology of the human OB and cerebral olfactory areas using a within-subject design with a comparable control group. In line with our preregistered hypothesis, we observed a consistent decrease of OB volume in 7 of 8 measured individuals within the C19+ group with an average of about a 14% decrease in volume at an average of 7 months after their infection by SARS-CoV-2. In the control group, 6 had an increase, and 6 had a decrease with time, with an average decrease of about 2% in OB volume. Although this was not a statistically significant difference between groups, according to the preregistered inference analyses, it is interesting to note that a binominal test indicates a medium effect size that is unlikely to occur due to chance, even when including the deviating participant with a large increase in OB volume. Thus, it can be speculated whether the lack of clear significant effects according to our preregistered analyses plan is mainly due to the small sample size, regulated by the unique and restricted population and the outlier participant.
Although multiple past studies have demonstrated that OB volume is modulated by changes in olfactory performance,24 the mechanism allowing this plasticity is not known. Studies in animal models have demonstrated that neurogenesis can occur in the OB,25 but studies in human cadavers have not supported this phenomenon. A more straightforward mechanism that might explain the link between the fast changes in OB volume that olfactory training is known to induce is potential changes in the OB vascularization. Recent data suggest that nearly all individuals infected by SARS-CoV-2 experience endothelial cell death, which causes microvascular damage to tissue along the olfactory pathway.9,26 Given the flexibility of the OB vascularization and close link to the amount of olfactory input,27 olfactory training might help alleviate OB morphologic loss due to COVD-19. However, we cannot dissociate between direct effects from SARS-CoV-2 and effects from a potential reduction in sensory input. Nevertheless, although several participants in the C19+ group reported subjective changes in olfactory functions, we did not find any statistically significant changes in objective olfactory performance.
We speculate that the participants’ self-reported olfactory problems might be parosmia-related, which is not well captured by the threshold-discrimination-identification test. For example, the ability to identify and discriminate odors is not necessarily affected by a change in their perceived nature and valence. Many individuals with parosmia will readily identify an odor, for example coffee, but will report that they no longer appreciate the odor and that coffee now smells more like smoke. We did not specifically interview participants about parosmia symptoms nor test for such, but 1 participant spontaneously shared that some odors had changed character (eg, sweat smelling like raw onions). Another participant even reported a constant strange stale taste in the mouth, a potential sign of phantosmia or phantgeusia. Another possibility is that our C19+ participants had specific anosmia (not coinciding with the 16 identification test odors). For example, 1 C19+ participant was surprised that he or she did not detect some of the odors in the discrimination task and did not recall having experienced this in the prestudy. Because this selective anosmia occurred only for, at most, 1 odor in each set for this participant, it did not impede the participant from performing the task. In fact, the discrimination task may even become easier when one of the odors in a set is odorless to the participant because they will, for example, easily detect the odd one out as being the only one that is odorless. Last, participants might have taste alterations that are easily confused with olfactory alterations, something that we did not assess in our study. One participant did report sensing more bitterness, and another reported poor taste, especially on the tip of the tongue, both of which might be signs of dysgeusia.
We found no evidence that a COVID-19 infection causes long-term insult to cerebral areas of the olfactory system, but we did demonstrate a significant increase of functional connectivity between the orbitofrontal cortex and both the anterior and posterior piriform cortex. These outcomes were not expected according to our hypothesis. However, absence of clear COVID-19-related morphologic changes to the piriform cortex, often referred to as the primary olfactory cortex, was also demonstrated in a recent study on the UK-biobank material in which pre- and post-COVID-19 infection data were included.28 In contrast, the UK-Biobank study found COVID-19-related reduction in GM within the orbitofrontal cortex, whereas we found no differences. The 2 studies differed in sample size, scanning parameters, and location of our olfactory-related ROIs. Nonetheless, our lack of significant results supports the emerging consensus that COVID-19 does not cause long-term morphologic alterations to the olfactory cortex of such magnitude that it can be clearly demonstrated, on average, 7 months after the infection. Although we did not expect an increased connectivity between the orbitofrontal cortex and the piriform cortex, our data clearly indicated that the C19+ group had a significantly higher functional connectivity between these 2 areas compared with the C19− group at the postmeasure. The piriform cortex is the largest cortical recipient of afferent OB fibers, whereas the orbitofrontal cortex (higher order multimodal sensory cortex) encodes information regarding olfactory stimuli (secondary olfactory functional area).
A recent study by Esposito et al29 evaluating olfactory loss and connectivity of the olfactory cortex after COVID-19 found that structural and functional connectivity metrics were significantly increased in participants with a previous COVID-19 infection compared with noninfected participants. As pointed out by these authors, limitations of their study included a small sample size as well as no data on pre-COVID olfactory performance. In addition, the authors did not perform resting-state fMRI assessment of the auditory or visual cortex (control regions) but focused only on the olfactory cortex. Although the authors could not exclude that the observed differences between the 2 groups (previously infected versus noninfected participants) might already have existed before the pandemic, they hypothesized that the observed increased functional connectivity of the olfactory cortex may be the result of a compensatory CNS response. Our study suggests that the observed increased connectivity between the piriform and orbitofrontal cortexes may indeed reflect a mechanism of CNS neuroplasticity, in particular because we found no significant group difference in connectivity in the 2 control regions, the auditory and visual cortexes.
The only C19+ patient who presented with a dramatic increase in OB volume reported parosmia symptoms. The increase in OB volume observed in this participant is probably caused by persistent localized edema and inflammation following infection. While transient bilateral edema of the OB has been described on MR images in C19+ patients during the acute phase of infection,13 a subset of patients presented with persistent olfactory deficits with or without perceptual distortions after COVID-19 infection.30 MR imaging, clinical, histopathologic, and molecular data suggest that in this subset of patients, localized inflammation of the olfactory pathways is responsible for the persistent olfactory deficits.30
The present study is in many ways unique in that we assessed the effects of COVID-19 infection within subjects, with a matching control group and using a study designed for assessing the potential neural effects of olfactory dysfunction. Without baseline pre-COVID-19 assessment or a control group, effects could be population-wide or reflect pre-existing COVID-19 risk factors. Nevertheless, our study is limited by the restricted sample size and does not have the same predicted power as a randomized control study. However, our baseline measures of the individual’s state before infection and, critically, the inclusion of individuals with only mild-to-moderate COVID-19 symptoms are a strength over studies assessing clinical cases with more severe symptoms in which the incidence of olfactory dysfunction is known to be much lower.31 Our cohort could potentially provide further unique data in the future by allowing assessment of tentative altered OB volume and connectivity in individuals experiencing either spontaneous or olfactory training-induced recovery.
Evidence from both animal and human data have demonstrated that a range of DNA and RNA viruses are first detected in the OB during neurotropic infections of the CNS.6 In line with this notion are recent data suggesting that though wide-spread disease-associated microglia signatures are found in COVID-19-infected patients’ cortexes, there are no molecular traces of SARS-CoV-2 in the cortex beyond the OB,32 a finding supported by the discovery of SARS-CoV-2 in the olfactory bulb but not beyond in an animal model.7 These findings are further in line with past data suggesting that OB interneurons are not affected by neurotropic coronaviruses33 and that the OB might provide virologic control by clearing viruses rapidly after infection.34 Our results of tentative long-term morphologic effects in the OB, but not the olfactory cortex, therefore support the notion that the OB is functioning as an immunosensory effector organ during neurotropic viral infections.6 Although our goal was not to assess the immediate clinical relevance and therapeutic significance of neuroimaging findings, our study may contribute to our understanding of the olfactory system. Critically, the understanding that OB volume is likely affected by COVID-19 further promotes the notion that clinicians should recommend olfactory training to patients with COVID-19 with lingering olfactory disturbances, given that olfactory training is known to affect OB volume.24
CONCLUSIONS
We found tentative evidence that COVID-19 reduces the volume of the OB with an average of 14% but does not affect GM volume of the main cerebral olfactory areas. Although 87.5% of our participants demonstrated a reduced OB volume after COVID-19 and binomial testing suggests that the result is not due to chance, our findings did not reach formal statistical significance.
Footnotes
Funding was provided by grants awarded to J.N. Lundström from the Knut and Alice Wallenberg Foundation (KAW 2018.0152), the Swedish Research Council (2021-06527), and a donation from Stiftelsen Bygg-Göta för Vetenskaplig forskning. Data acquisition was supported by a grant to the Stockholm University Brain Imaging Center (SU FV-5.1.2-1035-15).
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received May 14, 2022.
- Accepted after revision October 11, 2022.
- © 2022 by American Journal of Neuroradiology