Abstract
BACKGROUND AND PURPOSE: Transient loss of consciousness is commonly evaluated in the emergency department. Although typically caused by epileptic seizure, syncope, or psychogenic nonepileptic seizure, the underlying etiology is frequently misdiagnosed. Lateral tongue bites are reportedly a specific clinical finding of seizure. We have observed tongue signal abnormality suggesting bite injury on brain MR imaging after seizures. We hypothesized an association between tongue signal abnormality and seizure diagnosis among patients in the emergency department imaged for transient loss of consciousness. Our purposes were to determine the prevalence of tongue signal abnormality among this population and the predictive performance for seizure diagnosis.
MATERIALS AND METHODS: For this retrospective study including 82 brain MR imaging examinations, 2 readers independently assessed tongue signal abnormality on T2-weighted and T2-weighted FLAIR images. Discrepancies were resolved by consensus, and interrater reliability (Cohen κ) was calculated. The final diagnosis was recorded. Proportions were compared using the Fisher exact test.
RESULTS: Tongue signal abnormality was present on 19/82 (23%) MR imaging examinations. Interrater reliability was “substantial” (κ = 0.77). Seizure was diagnosed among 18/19 (95%) patients with tongue signal abnormality and 29/63 (46%) patients without it (P < .001). In our cohort, tongue signal abnormality conveyed 97% specificity, 95% positive predictive value, and 63% accuracy for seizure diagnosis.
CONCLUSIONS: Tongue signal abnormality was observed in 23% of the study cohort and conveyed 97% specificity and 95% positive predictive value for seizure diagnosis. By assessing and reporting tongue signal abnormality, radiologists may facilitate a timely and accurate diagnosis of seizure among patients imaged for transient loss of consciousness.
ABBREVIATIONS:
- ED
- emergency department
- ES
- epileptic seizure
- PNES
- psychogenic nonepileptic seizure
- TLoC
- transient loss of consciousness
- TSA
- tongue signal abnormality
Transient loss of consciousness (TLoC) is defined as a spontaneous, temporary loss of consciousness with complete recovery.1 TLoC is estimated to affect up to 50% of individuals at some point in their lives1 and to account for up to 3% of emergency department (ED) visits.2 Although >90% of cases of TLoC are known to be caused by epileptic seizure (ES), syncope, or psychogenic nonepileptic seizure (PNES),3 confident determination of the underlying etiology in any given patient remains difficult. In fact, it is estimated that the underlying cause of TLoC is misdiagnosed in 20%–30% of cases.4⇓⇓-7 A timely and accurate diagnosis of seizure identifies patients with TLoC who may benefit from antiepileptic therapy, whereas an incorrect diagnosis may result in inaccurate, inefficient, and delayed care.
The terminology associated with 2 of the most common causes of TLoC (ES, PNES) is potentially confusing. Thus, clarification is offered on the use of ES and PNES throughout this article. The International League Against Epilepsy defines ES as “a transient occurrence of signs and/or symptoms due to abnormal excessive or synchronous neuronal activity in the brain.”8 Most important, the term ES—commonly shortened to seizure—does not imply that the seizure is caused by or the patient has an epilepsy syndrome. Rather, the intent is to differentiate ES from other physical or psychological sudden events that may resemble ES in some ways but which have causes other than abnormal excessive or synchronous neuronal activity in the brain,8 for example PNES, “an event resembling ES but caused by psychological processes”9 and historically also referred to as “pseudoseizure.”
Lateral tongue bites have been described as a specific clinical finding of ES.10⇓⇓⇓-14 Some data suggest that bite injuries limited to the tip of the tongue are specific for syncope;10,15,16 however, this pattern of injury has also been observed in ES11 and PNES.14 On brain MR imaging, we have observed tongue signal abnormality (TSA), which was subsequently proved to correspond to tongue bite injury and was used to support a clinical diagnosis of unrecognized seizure. Neuroimaging is commonly performed in the evaluation of TLoC,17 and the presence of TSA may implicate ES as the underlying etiology. We hypothesized a positive association between TSA and the clinical diagnosis of ES. The purposes of this study were the following: 1) to determine the prevalence of TSA among patients in the ED undergoing brain MR imaging for TLoC, and 2) to assess associations between TSA and a final clinical diagnosis of seizure.
MATERIALS AND METHODS
Subjects
For this retrospective, Health Insurance Portability and Accountability Act–compliant, institutional review board–approved study, a local institutional radiology database (mPower; Nuance Healthcare) was queried for patients satisfying the following inclusion criteria: 1) 18 years of age or older, 2) evaluated in our institution’s ED between May 2016 and May 2020, and 3) brain MR imaging obtained during ED evaluation including the terms “seizure,” “syncope,” “loss of consciousness,” or “fainting” in the study indication. Examinations were excluded if neither the T2-weighted nor T2-weighted FLAIR images included the tongue or the diagnostic assessment of the tongue was precluded by severe motion, dental, or other imaging artifacts.
Medical Record Review
Patient age and sex, stated indication for imaging, the presence or absence of clinical documentation of tongue bite injury, and the final clinical diagnosis (eg, seizure, syncope, other) were recorded.
Image Acquisition
Given the retrospective nature of this study, there was variability with respect to the MR imaging scanners used to acquire images and the specific T2-weighted and T2-weighted FLAIR sequence acquisition parameters. Most included MR imaging examinations were performed on 1 of three 1.5T MR imaging scanners: Magnetom Avanto (n = 45) (Siemens), Magnetom Aera (n = 17) (Siemens), and Signa HDxt (n = 5) (GE Healthcare). The remaining 15 included MR imaging examinations were performed on 1 of two 3T Magnetom Skyra (Siemens) MR imaging scanners. Representative T2-weighted acquisition parameters for the most commonly used 1.5T MR imaging scanner were the following: 5-mm section thickness, 5-mm spacing, FOV = 230 × 230 mm, matrix = 320 × 320, NEX = 1, TR = 4200 ms, TE = 105 ms, echo-train length = 35. Representative T2-weighted FLAIR acquisition parameters applied to the most commonly used 1.5T MR imaging scanners were the following: 5-mm section thickness, 7-mm spacing, FOV = 230 × 230 mm, matrix = 256 × 223, NEX = 1, TR = 8000 ms, TE = 120 ms, TI = 2370 ms, echo-train length = 16.
Reader Assessment
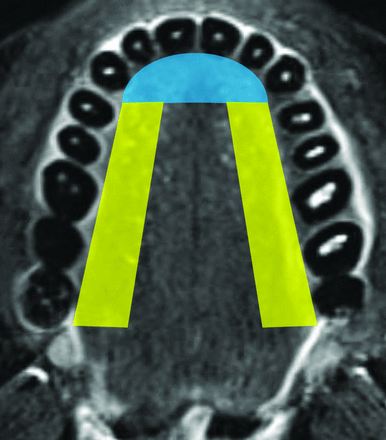
One radiology resident and 1 fellowship-trained attending neuroradiologist (4 years’ subspecialty experience), both blinded to the clinical information, independently reviewed the T2-weighted and T2-weighted FLAIR images from all included brain MR imaging examinations using our institution’s PACS. Each reviewer recorded the presence or absence of TSA in all patients, defined as abnormally increased fluid signal involving the tongue. For TSA identified on non-fat-suppressed T2-weighted FLAIR images, correlation with corresponding T1-weighted images of the tongue was performed to confirm that the TSA was consistent with fluid signal rather than fat. The recorded results were compared, and discrepancies were resolved by consensus review. Consensus review was performed unblinded to the clinical documentation of the presence or absence of tongue bite injury on physical examination. When a TSA was present, the radiology report was reviewed to determine whether the finding was described. Additionally, both reviewers in consensus characterized the site of TSA as “lateral tongue,” “tip of tongue,” or “both” using a visual standard (Fig 1) modeled after the visual depiction of the bite location by Benbadis et al.10
Axial, T2-weighted, fat-suppressed image with color overlay demonstrates the visual standard used to classify the sites of tongue signal abnormality as involving the tip of tongue (blue shading), lateral tongue (yellow shading), or both.
Statistical Analysis
Absolute and relative frequencies are reported for categoric variables. The Fisher exact test was used to compare proportions, the Student t test was used to compare continuous variables, and the Cohen κ coefficient was calculated to assess interrater reliability. These analyses were performed with JMP, Version 14 (SAS Institute), and P < .05 indicated a statistically significant difference. Measures of diagnostic performance (eg, sensitivity, specificity, positive predictive value) for TSA and clinically documented tongue bite injury were also calculated.
RESULTS
Subjects
A total of 144 brain MR imaging examinations were reviewed, with 55 examinations excluded because the tongue was not included in the scan range of either the T2-weighted or the T2-weighted FLAIR sequences and 7 excluded for artifacts (eg, motion, dental) precluding assessment of the tongue. This process yielded a total of 82 brain MR imaging examinations in the cohort. Characteristics of the study group are summarized in Table 1.
Characteristics of the study group
Reader Assessment
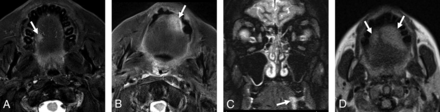
Following consensus review, TSA was determined to be present on 19 (23%) MR imaging examinations (Fig 2), more commonly unilateral (11/19; 58%) than bilateral (8/19; 42%). The site of the TSA was classified as the lateral tongue only in 12 (63%) patients, tip of tongue only in none (0%), and both the lateral tongue and tip of tongue in 7 (37%) patients. There were no significant differences between the presence of TSA and age (P = .22) or sex (P = .80). Interrater reliability was substantial (κ = 0.77). The T2-weighted and T2-FLAIR weighted sequences on which the tongue was evaluable are summarized in Table 2.
Representative examples of the spectrum of TSA (arrows, A–D) observed in this study from 4 different patients in the ED. All patients were given a final clinical diagnosis of epileptic seizure. Tongue bite injuries were documented on physical examination for the patients depicted in B and D. No tongue bite injury was documented on physical examination for the patients depicted in A and C. TSA was classified as lateral only for A and C and as both lateral and tip of tongue for B and D.
Summary of T2-weighted and T2-weighted FLAIR sequences on which the tongue was evaluable within the study cohort and visibility of TSA by sequence
TSA was observed in 12/15 (80%) patients with documented tongue bite injuries on physical examination and in 7/67 (10%) patients with no documented tongue bite injury on physical examination (P < .001). Among the 15 patients with TSA and documented tongue bite injuries, the sites of the TSA corresponded with the sites of documented tongue bite injuries in all (3/3) patients for whom specific site information (eg, right, left, bilateral) was documented; in the other 12 patients, physical examination documented only “tongue bite injury” or “tongue laceration” with no specification as to sites of injury. The final clinical diagnosis was ES among 18/19 (95%) patients with TSA (11 classified as involving the lateral tongue only and 7 classified as involving both the lateral tongue and tip of tongue) and 29/63 (46%) patients without TSA (P < .001). One patient with TSA and a clinically documented tongue bite injury (classified as involving the lateral tongue only) was given a final clinical diagnosis of “spells of altered attention, likely cognitive fluctuations in the setting of dementia.” The final clinical diagnosis was ES for all 7 patients with TSA but no documented tongue bite injury on physical examination.
In our cohort, TSA conveyed 38% sensitivity (95% CI, 25%–54%), 97% specificity (95% CI, 85%–100%), 95% positive predictive value (95% CI, 72%–99%), 54% negative predictive value (95% CI, 48%–60%), and 63% accuracy (95% CI, 52%–74%) for a final clinical diagnosis of ES. TSA was described in 0/19 radiology reports.
For comparison, the final clinical diagnosis was ES among 14/15 (93%) patients with documented tongue bite injury on physical examination compared with 33/67 (49%) patients without documented tongue bite injury (P = .001). In our cohort, documented tongue bite injury conveyed 30% sensitivity (95% CI, 17%–45%), 97% specificity (95% CI, 85%–100%), 93% positive predictive value (95% CI, 66%–99%), 51% negative predictive value (95% CI, 46%–56%), and 59% accuracy (95% CI, 47%–69%) for a final clinical diagnosis of ES.
DISCUSSION
Among patients in the ED undergoing brain MR imaging for TLoC, the overall prevalence of TSA suggesting bite injury was 23%, and the underlying etiology for TLoC was determined to be ES in 95% of patients with TSA. Notably, the final clinical diagnosis was seizure in 100% of patients with TSA but no documented tongue bite injury on physical examination, suggesting that either the physical examination in the ED overlooked a tongue bite injury in these patients or the injury was not apparent at the time of mucosal inspection. Moreover, no interpreting radiologist described the presence of TSA in any of this cohort’s brain MR imaging reports, suggesting that radiologists are not habitually assessing the tongue for possible bite injury on brain MR imaging performed for TLoC.
These findings support our hypothesis that TSA—presumed to represent the MR imaging correlate to tongue bite injury—is positively associated with the clinical diagnosis of seizure. Both TSA and clinically documented tongue bite injuries conveyed high specificity (97% for both) and high positive predictive values (95% for TSA, 93% for tongue bite) for a final clinical diagnosis of ES. Most important, the absence of TSA does not preclude seizure as the underlying etiology for TLoC because the sensitivity of TSA for clinical seizure diagnosis was only 38%. Diagnostic performance of TSA for seizure diagnosis was comparable with that of tongue bite injuries both in our cohort and in the published literature, including a meta-analysis reporting a pooled sensitivity of 33% and pooled specificity of 96% for tongue bite injury.13
TSA location (lateral, tip of tongue, both) was also assessed, given a previous pooled analysis of data from Benbadis et al10 and Akor et al15 reporting 99.8% specificity of a tip of the tongue bite for syncope.16 There were no patients in our cohort with TSA limited to just the tip of tongue. However, TSA involved both the tip of tongue and the lateral tongue in 7 patients, all of whom were diagnosed with ES. Given reports of tip of the tongue bites in both ES11 and PNES14 as well as the pooled analysis from Brigo et al16 including only 2 patients with syncope with tip of tongue bites, we advise caution in drawing conclusions about the underlying cause of TLoC on the basis of tip of tongue bite location alone.
TLoC accounts for up to 3%2 of all ED visits, and the underlying cause of TLoC is misdiagnosed in 20%–30% of cases,4⇓⇓-7 potentially resulting in inaccurate, inefficient, or delayed care. When interpreting neuroimaging in patients with TLoC, the radiologist should be primarily concerned with evaluating for mass, hemorrhage, infarction, encephalitis, or other structural causes for TLoC. However, radiologists’ awareness of, assessment for, and reporting of TSA as a high-specificity finding for ES could facilitate timely identification of patients who may benefit from antiepileptic therapy, particularly given that some tongue bite injuries may be unrecognized on physical examination in the ED setting and that some patients may undergo imaging before comprehensive oral cavity examination. Thus, assessment of the tongue on brain MR imaging may complement clinical history, physical examination, witness accounts,18 electroencephalography, electrocardiography, heart rhythm monitoring, tilt-table testing, and laboratory evaluation19⇓-21 to ascertain underlying TLoC etiology and, in some cases, potentially obviate the need for more involved additional tests. We do not advocate obtaining brain MR imaging in all patients with TLoC to enable TSA assessment. However, in the subset of patients with TLoC whose clinical circumstances warrant brain MR imaging, radiologists have an opportunity to facilitate an accurate diagnosis of seizure by noticing TSA, reporting its presence, and recommending correlation with direct inspection to confirm that the finding indeed represents a tongue bite injury rather than mucosal neoplasm or other potential mimics.
Some radiology practices might consider routinely including the tongue within the scan range of brain MR imaging performed for TLoC (if not always included already) to enable assessment for TSA; however, such a protocol change would likely need to be considered in the context of local practice patterns, radiologists’ and referring providers’ preferences, and other factors beyond the scope of this study. Although there were no statistically significant differences between the frequencies with which TSA was observed on fat-suppressed-versus-non-fat-suppressed images (Table 2), in our subjective experience, TSA was more easily and more confidently identifiable on the fat-suppressed images. We found both axial and coronal fat-suppressed T2-weighted and T2-weighted FLAIR images equally valuable for TSA identification. Thus, if considering protocol modifications to maximize the potential usefulness of TSA in the imaging of TLoC, we suggest including the tongue in the scan range of at least 1 fat-suppressed T2-weighted or T2-weighted FLAIR sequence. Alternatively, if one is assessing non-fat-suppressed T2-weighted or T2-weighted FLAIR images for TSA, we emphasize the importance of correlating any findings on the T2-weighted sequences with T1-weighted images to avoid misdiagnosing focal fat (T1 and T2 hyperintense) as TSA (T1 hypointense, T2 hyperintense).
There are limitations to this study. The retrospective design precludes definitive confirmation that TSA represented tongue bite injury in all patients; however, the statistically significant association between the presence of TSA and a documented tongue bite injury, the parallel between the site of TSA and the site of documented bite injury in all patients for whom this level of clinical detail was available, as well as the comparable diagnostic performance of both findings for clinical seizure diagnosis provide evidence that the TSA observed in our study is indeed the MR imaging correlate to tongue bite injury. A substantial proportion of brain MR imaging examinations in the ED performed for TLoC did not include images of the tongue and were, therefore, excluded. Furthermore, the criterion standard of final clinical diagnosis used in this study is known to be imperfect. The real-world clinical impact of radiologists’ identification of TSA is uncertain and would likely depend on a number of factors not controlled for in this study, including the following: 1) whether an oral cavity examination was performed before MR imaging, 2) the training and skill level of the oral cavity examiner, 3) whether a tongue bite injury was already identified before imaging, and 4) clinical decision-making related to which patients with TLoC are selected for brain MR imaging. Finally, only 2 patients in the cohort had a final diagnosis of PNES, so any potential relationship between TSA and PNES remains uncertain.
CONCLUSIONS
The prevalence of TSA among patients in the ED undergoing brain MR imaging for TLoC was 23%, and the presence of TSA conveyed 97% specificity and 95% positive predictive value for a final clinical diagnosis of ES.
Radiologists have an opportunity to add value in the MR imaging evaluation of TLoC through awareness of, assessment for, and reporting of TSA as a high-specificity finding for ES, particularly given that some tongue bite injuries may be unrecognized on physical examination in the ED setting.
Footnotes
Paper previously presented as an electronic scientific poster at: Annual Meeting of the American Society of Neuroradiology. May 20 to June 4, 2020; Virtual. The current submission includes additional data and analyses that were not part of the previous electronic poster.
References
- Received January 14, 2021.
- Accepted after revision April 4, 2021.
- © 2021 by American Journal of Neuroradiology