Abstract
BACKGROUND AND PURPOSE: Task-based fMRI is a noninvasive method of determining language dominance; however, not all children can complete language tasks due to age, cognitive/intellectual, or language barriers. Task-free approaches such as resting-state fMRI offer an alternative method. This study evaluated resting-state fMRI for predicting language laterality in children with drug-resistant epilepsy.
MATERIALS AND METHODS: A retrospective review of 43 children with drug-resistant epilepsy who had undergone resting-state fMRI and task-based fMRI during presurgical evaluation was conducted. Independent component analysis of resting-state fMRI was used to identify language networks by comparing the independent components with a language network template. Concordance rates in language laterality between resting-state fMRI and each of the 4 task-based fMRI language paradigms (auditory description decision, auditory category, verbal fluency, and silent word generation tasks) were calculated.
RESULTS: Concordance ranged from 0.64 (95% CI, 0.48–0.65) to 0.73 (95% CI, 0.58–0.87), depending on the language paradigm, with the highest concordance found for the auditory description decision task. Most (78%–83%) patients identified as left-lateralized on task-based fMRI were correctly classified as left-lateralized on resting-state fMRI. No patients classified as right-lateralized or bilateral on task-based fMRI were correctly classified by resting-state fMRI.
CONCLUSIONS: While resting-state fMRI correctly classified most patients who had typical (left) language dominance, its ability to correctly classify patients with atypical (right or bilateral) language dominance was poor. Further study is required before resting-state fMRI can be used clinically for language mapping in the context of epilepsy surgery evaluation in children with drug-resistant epilepsy.
ABBREVIATIONS:
- ACT
- auditory category task
- ADDT
- auditory description decision task
- DRE
- drug-resistant epilepsy
- IC
- independent component
- ICA
- independent component analysis
- LI
- laterality index
- rs-fMRI
- resting-state fMRI
- SWG
- silent word generation
- tb-fMRI
- task-based fMRI
- VF
- verbal fluency.
Pediatric drug-resistant epilepsy (DRE) is defined as poorly controlled seizures despite treatment on ≥2 appropriately used antiseizure medications.1 Surgery is recommended when seizures have not responded to treatment with antiseizure medications. Surgery could result in seizure freedom in up to 90% of children with DRE.2,3 The risk of language impairment is a key consideration in determining surgical candidacy and surgical planning. In neurologically intact individuals, language is supported by a predominantly left-lateralized frontal-temporal network. Up to 25% of children and adults with epilepsy have atypical language dominance (ie, language lateralized to the right hemisphere or bilaterally across both the left and right hemispheres) compared with only 3% of healthy children and adults.4 Hence, determining hemispheric language dominance during presurgical evaluation is critical in preventing postsurgical language deficits in children with DRE.
The intracarotid amobarbital procedure, previously known as the Wada test, is currently the criterion standard for determining language laterality. Electrical stimulation mapping is also considered the criterion standard for functional localization. More recently, however, task-based fMRI (tb-fMRI) has been commonly used for establishing language laterality in clinical practice because it is less invasive and carries less risk. Tb-fMRI has shown good concordance with the intracarotid amobarbital procedure, with concordance rates of 87% and 81% in adults with medial-temporal and extratemporal epilepsy, respectively.5 Concordance rates in pediatric epilepsy have been slightly lower, with a recent meta-analysis of 21 studies finding an overall concordance rate between the intracarotid amobarbital procedure and tb-fMRI of 76% and sensitivity and specificity of 0.72 and 0.60, respectively, in correctly classifying typical-versus-atypical language lateralization.6 However, not all children are suitable for tb-fMRI. Children who are very young or have cognitive/intellectual disabilities cannot always complete the tasks necessary for tb-fMRI. Task-free approaches such as resting-state fMRI (rs-fMRI) may offer an alternative to task-based approaches, especially for children in whom tb-fMRI is contraindicated.
Preliminary evidence suggests that rs-fMRI is promising for lateralizing language in healthy7,8 and clinical populations of adults with tumor9,10 and epilepsy,11,12 though concordance rates are highly variable across studies. Only 2 studies have examined the use of rs-fMRI to determine language dominance in children with epilepsy compared with tb-fMRI,13,14 and these 2 studies were limited by the use of visual assessment for classifying language laterality and/or small sample size, and in 1 study, bilateral language lateralization was excluded. This study aimed to evaluate the validity of rs-fMRI in predicting language laterality compared with tb-fMRI in children with DRE.
MATERIALS AND METHODS
Participants
This retrospective study included 51 children with DRE (7–18 years of age) who were undergoing epilepsy surgery evaluation at the Hospital for Sick Children, Toronto, Ontario, Canada. We excluded 8 children: Seven did not have tb-fMRI, and in 1 case, the rs-fMRI data were excluded due to motion. This study included 43 children who had data on rs-fMRI and at least 1 of 4 language tb-fMRI paradigms. Ethics approval was obtained from the local research ethics board. The baseline characteristics of the sample are shown in the Online Supplemental Data.
MR Imaging
MR imaging was performed using a 3T scanner (Achieva, Philips Healthcare, n = 27, or Magnetom Skyra, Siemens, n = 16). The rs-fMRI and tb-fMRI were acquired using gradient EPI. The scan parameters for rs-fMRI and tb-fMRI on the Achieva scanner were the following: TR = 2000 ms, TE = 30 ms, flip angle = 90°, FOV = 220 mm, voxel size = 2.5 × 2.5 × 3.5 mm, and 180 volumes. The scan parameters for rs-fMRI and tb-fMRI on Magnetom Skyra were the following: TR = 2000 ms, TE = 30 ms, flip angle = 90°, FOV = 220 mm, voxel size = 2.3 × 2.3 × 2.0 mm, and 180 volumes. Rs-fMRI required children to lie still with their eyes closed for the duration of the 6-minute scan. All patients also underwent volumetric T1-weighted imaging. Rs-fMRI was acquired before the tb-fMRI.
A block design consisting of alternating 30-second blocks of experimental and control conditions was used. Each task consisted of 12 blocks (6 task blocks and 6 control blocks) with a total task time of 6 minutes. Auditory stimuli were presented via headphones. Visual stimuli were presented via MR imaging goggles.
Tb-fMRI Language Paradigms
The tb-fMRI involved 4 standard language paradigms: verbal fluency (VF), silent word generation (SWG), auditory description decision task (ADDT), and auditory category task (ACT) (Table 1).
Language task-based fMRI tasks
Rs-fMRI Data Preprocessing
Rs-fMRI images were processed with FSL software (http://www.fmrib.ox.ac.uk/fsl). Nonbrain regions including the skull were removed from all structural T1 images by the FSL Brain Extraction Tool (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/BET)15 with a fractional intensity threshold of 0.1. Images were motion-corrected using the FSL motion correction of functional images using the Linear Image Registration Tool (FLIRT; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FLIRT).16 Data were scrubbed by removing images showing root mean square relative displacement of >0.25 mm or root mean square absolute displacement of >2.5 mm.17 All scrubbed data were post-processed using the MELODIC 3.0 tool (https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/MELODIC).18 To eliminate noise due to slow temporal drifts, we used a high-pass filter with the default cutoff value 100 seconds (0.01 Hz). Spatial smoothing was performed by the full width at half maximum Gaussian kernel of 5 mm. All functional images were linearly coregistered to the structural image using FLIRT16,19 affine transformation with 7 degrees of freedom (df). Later, spatial registration of fMRI to the high-resolution standard Montreal Neurological Institute 152 T1 template was performed with 12 df.
Independent Component Analysis
Single-subject independent component analysis (ICA) was performed to extract language networks from resting-state data. For each subject, the maximum (mean = 51 [SD, 10]) possible independent components (ICs) were extracted. IC spatial maps were thresholded with the alternative hypothesis tested at a voxel-based P value > .5 for true activation (signal) versus null (noise). An automated algorithm was used to select “signal” from “noise” ICs. First, an expert (N.L.P.) manually hand-labeled IC maps from 23 subjects as signal or noise based on overlap with gray matter, number, and dimensions of clusters; extent of overlap with brain boundaries; and temporal features of the ICs.20 Labeled signal ICs were then used to train the FMRIB ICA-based X-noiseifier (FIX; https://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FIX) hierarchical classifier.21,22 Using the study-specific training dataset, we chose a threshold of 40 for categorizing the ICs in the remaining 20 subjects, accurately based on 2 accuracy parameters: the highest true-positive rate (proportion of signal components correctly labeled) and the lowest true-negative rate (proportion of noise components correctly labeled). The ICs that were selected as signal by the algorithm were visually inspected to confirm that they were signal ICs.
To select the language IC from denoised data, we calculated9 Dice coefficients—ie, 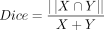 were used to measure the degree of overlap between ICs and language regions, including the inferior frontal gyrus, middle temporal gyrus, superior temporal gyrus, angular gyrus, and supramarginal gyrus, identified using the Willard atlas.23,24 The IC with the highest Dice coefficient (range, 0–1, with 1 indicating the greatest spatial overlap) was selected as the language IC for each participant. These components were then converted into z score maps using the following thresholds: z = 1.5, 2.5, and 3 (ie, P = .05, .01, and .001). Visual identification of noise components and subsequent identification and ranking of language ICs were conducted by 2 authors (N.L.P. and E.W., κ = 0.84), to validate and ensure the accuracy of the automated procedures described above.
were used to measure the degree of overlap between ICs and language regions, including the inferior frontal gyrus, middle temporal gyrus, superior temporal gyrus, angular gyrus, and supramarginal gyrus, identified using the Willard atlas.23,24 The IC with the highest Dice coefficient (range, 0–1, with 1 indicating the greatest spatial overlap) was selected as the language IC for each participant. These components were then converted into z score maps using the following thresholds: z = 1.5, 2.5, and 3 (ie, P = .05, .01, and .001). Visual identification of noise components and subsequent identification and ranking of language ICs were conducted by 2 authors (N.L.P. and E.W., κ = 0.84), to validate and ensure the accuracy of the automated procedures described above.
Tb-fMRI Analysis
Tb-fMRI images were processed using Analysis of Functional Neuro Images (AFNI; http://afni.nimh.nih.gov/afni). Preprocessing included registering the raw EPI volumes to the EPI base volume, outlier detection and censoring outlier time points, and section-timing correction. The images were then smoothed with a 5.0-mm full width at half maximum Gaussian kernel. For each task, activation maps were generated using the FSL General Linear Model (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/GLM) analysis.
Language Laterality
The laterality index (LI) was calculated for rs-fMRI and tb-fMRI data using the standard formula, LI = (Left – Right) / (Left + Right),25 where Left and Right are the number of voxels in the left and right hemispheres in the given ROIs for each threshold, respectively. The LI values ranged from −1 (right-dominant) to +1 (left-dominant) with a cutoff of ±0.2. The LIs of selected ICs from rs-fMRI were calculated at a z-threshold (z = 1.5, 2.5, and 3). The LI for tb-fMRI was first calculated at 3 thresholds: t = 2 (P = .05), t = 2.5 (P = .01), and t = 3.5 (P = .001). If laterality differed across the 3 thresholds for a task, the most common classification was chosen (eg, if a subject was classified as bilateral on the ADDT language paradigm at t = 2 but left-lateralized at t = 2.5 and t = 3.5, he or she was classified as left-lateralized for the ADDT paradigm). This approach is consistent with that of a previous study.26
Statistical Analysis
Data were analyzed using SPSS Statistics (Version 26.0; IBM). Concordance between the 4 tb-fMRI language paradigms and rs-fMRI at each threshold was calculated using the following: 1) descriptive statistics (frequencies) by laterality (left, right, bilateral), and 2) the overall agreement rate with 95% CIs. Subgroup analyses were conducted comparing the agreement between rs-fMRI and tb-fMRI based on scanner type, age (younger than 13 years of age or 13 years of age or older), sex, side of seizure onset, and handedness. The agreement between the LI and visual inspection of rs-fMRI language hemispheric dominance was also assessed.
RESULTS
Language laterality findings for tb-fMRI and rs-fMRI are presented in Table 2.
Language laterality based on resting-state and task-based fMRI
Across the 4 language tasks, tb-fMRI suggested left dominance in 80%–90%, right dominance in 2% to 5%, and bilateral language dominance in 8%–14% of patients. Rs-fMRI suggested left dominance in 79%–84%, right dominance in 7%, and bilateral language dominance in 9%–14% of patents, depending on the z-threshold.
The Online Supplemental Data show concordance between the 4 language tb-fMRI paradigms and rs-fMRI at each z-threshold. Overall, concordance rates for language laterality between rs-fMRI and tb-fMRI were highest for the ADDT paradigm and ranged from 0.73 (95% CI, 0.58–0.87) to 0.70 (95% CI, 0.55–0.85), with higher concordance rates found at rs-fMRI thresholds of z = 1.5 and z = 3, compared with z = 2.5. With respect to the ACT paradigm, concordance was highest with rs-fMRI at a threshold of z = 1.5, with an agreement rate of 0.70 (95% CI, 0.55–0.84). Concordance rates for the VF paradigm were the lowest of the tb-fMRI paradigms and ranged from 0.64 (95% CI, 0.48–0.80) to 0.65 (95% CI, 0.50–0.80). Last, concordance between the rs-fMRI and SWG paradigms, which was highest at the most stringent rs-fMRI threshold of z = 3, ranged from 0.69 (95% CI, 0.54–0.84) to 0.66 (95% CI, 0.52–0.8)
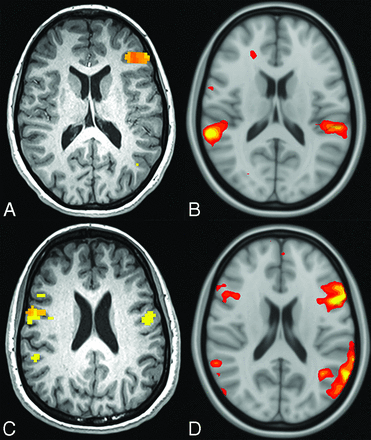
Between 78% and 83% of patients identified as left-lateralized on tb-fMRI were correctly classified as left-lateralized on rs-fMRI. In contrast, no patients identified as right-lateralized on tb-fMRI were correctly classified. Instead, all patients were classified as left-lateralized on rs-fMRI. No patients identified as bilateral on tb-fMRI were classified correctly. Most were classified as left-lateralized (80%–100%) (Figure), while the rest were classified as right-lateralized (0%–20%; Online Supplemental Data).
An 11-year-old child (A and B) with left-lateralized language on tb-fMRI and bilateral language on rs-fMRI. A 14-year-old adolescent (C and D) with bilateral language on tb-fMRI and left-lateralized language on rs-fMRI.
Subgroup analyses showed that there were no significant differences in agreement between rs-fMRI and tb-fMRI based on scanner type, age, sex, side of seizure onset, or handedness (all P > .05) (Online Supplemental Data).
Concordance between the LI and visual inspection of rs-fMRI language hemispheric dominance was highest at a threshold of z = 2.5; (0.79; 95% CI, 0.78–0.80), followed by z = 3 (0.73; 95% CI, 0.72–074) and lowest at z = 1.5 (0.66; 95% CI, 0.65–0.67). Four patients identified as left-lateralized on rs-fMRI LI were classified as bilateral on visual inspection.
DISCUSSION
This study examined the validity of rs-fMRI for predicting language laterality in children with DRE by comparing rs-fMRI with a panel of established tb-fMRI language paradigms. We found concordance rates of 64%–73% between rs-fMRI and tb-fMRI. While rs-fMRI correctly identified most patients with left-lateralization, it did not correctly classify those with bilateral or right dominance. A small group of patents was classified as having language dominance in the contralateral hemisphere on rs-fMRI compared with tb-fMRI. Specifically, all patients classified as right-lateralized for language on tb-fMRI were classified as left-lateralized on rs-fMRI, and those who were classified as left-lateralized on tb-fMRI were right-lateralized on rs-fMRI, albeit the number of cases was small.
Earlier studies13,14 involving pediatric DRE found higher concordance rates between rs-fMRI and tb-fMRI compared with our study. Desai et al13 found a concordance rate of 93% in 28 children with DRE. Rs-fMRI correctly classified 23 (92%) patients as left- and 3 (100%) patients as right-lateralized for language. However, the authors relied on visual inspection by a neuroradiologist to make a clinical judgment regarding the following: 1) identification of each patient’s rs-fMRI language networks extracted using ICA, and 2) classification of language laterality on tb-fMRI and rs-fMRI. Visual inspection of individual ICs is time- and labor-intensive and potentially less replicable. In contrast, we have developed an automated approach for selecting signal from noise ICs and then used a data-driven approach to select the IC that contained the language network by comparing our signal ICs with a standard language network template using the Dice coefficient measure. This approach is automated and can be consistently and objectively applied across studies and clinical samples. Nath et al14 found a concordance rate of 80% between rs-fMRI (using a seed-based approach to identify language networks) and traditional methods of language lateralization (tb-fMRI, intracarotid amobarbital procedure, or cortical-stimulation mapping) in children with epilepsy. Nevertheless, the sample size was small, and patients with bilateral language lateralization were excluded. Hence, the results do not extend to children with bilateral language lateralization. In addition, language lateralization for tb-fMRI was based on frontal (Broca) seed regions rather than frontal-temporal seed regions, and only 5 participants had tb-fMRI data, which was based on only 1 language paradigm (verb generation). This finding is important because language lateralization determined across several paradigms is considered more reliable, especially in those with bilateral lateralization.27
A recent study of adults with DRE11 found that language dominance was less lateralized on seed-based rs-fMRI data compared with tb-fMRI. The authors also found concordance rates of between 20% and 63%, with the highest concordance rate found when using a frontal-temporal mask (compared with just frontal or temporal) and at the top 10% threshold of connections.11 Furthermore, they showed that the method for calculating LI for rs-fMRI influenced the classification of language lateralization, with concordance of dominance classifications ranging from 20% to 30% for the intrahemispheric LI method and 50%–63% for the intrahemispheric-minus-interhemispheric difference LI method. We have used the commonly used intrahemispheric LI method to evaluate language laterality. While we found higher concordance rates using the intrahemispheric LI approach compared with Rolinski et al,11 our overall concordance rate was within a similar range. Rolinski et al also found that 40% of patients who were left-dominant on tb-fMRI showed bilateral language dominance on rs-fMRI. While we found similar numbers of bilateral language dominance on rs-fMRI relative to tb-fMRI, all of those that were found to have bilateral dominance on rs-fMRI were left-lateralized on tb-fMRI, and most who were bilateral-dominant on tb-fMRI were left-lateralized on rs-fMRI.
In our study, the concordance rate between rs-fMRI and tb-fMRI was variable, depending on the tb-fMRI language paradigm, with the highest concordance rates found for ADDT. Most previous studies have compared rs-fMRI with just 1 language paradigm.
However, different language paradigms have been shown to activate different brain regions, possibly accounting for the variable concordance rates. The ADDT is the most reliable activator of the frontal (inferior frontal gyrus, prefrontal cortex) and temporal (superior and middle temporal gyri) language networks,28 possibly explaining the higher concordance rate found in our study. In contrast, the remaining language paradigms were largely frontal- or temporal-dominant tasks. Our results highlight the need to validate rs-fMRI against a battery of tb-fMRI language paradigms in research as well as clinical practice because results may vary.
This study has important clinical implications for the use of rs-fMRI in presurgical planning for children with DRE. There is a growing trend of using rs-fMRI to lateralize language in patients who are not cooperative or may require light sedation. We found that there was greater agreement with typical language lateralization but high discordance for atypical language lateralization with tb-fMRI. Our findings suggest that rs-fMRI should not be used as the sole method for lateralizing language in uncooperative or sedated patients in clinical practice and should not replace tb-fMRI for lateralizing language. Further work is required to compare rs-fMRI language lateralization with the intracarotid amobarbital procedure and to validate our findings across institutions.
The 2 commonly used methods for analyzing rs-fMRI to lateralize language are ICA9,12,13,29 or seed-based11 approaches. ICA is data-driven and can be performed without any a priori assumptions. A disadvantage of the ICA approach is that individual IC maps may not depict the entirety of a network if the ICA order is too high—that is, splitting of a network into subnetworks tends to occur at a high ICA order. It is possible that different ICs depicted right- and left-lateral aspects of the language network. We have used the IC with the highest Dice coefficient as the language IC, which could potentially have failed to identify additional ICs that represent language subnetworks, thereby misclassifying language laterality. However, this approach has been used by several investigators for assessing language IC.3,12,29 Branco et al9 have extracted a mean of 66.3 ICs, which was higher than the number of ICs in our study, and showed that for each subject, 1 IC was identified as the language component with high confidence by an expert. They also found that there was good agreement between the IC with the highest ranked Dice coefficient and the expert-selected language component. The main advantage of a seed-based approach is that the computation is simple and the interpretation of the results is intuitive.30 However, the position of the seed region could affect the resulting patterns of the functional system such as the language network. Studies using a seed-based approach have reported lower concordance rates compared with those using the ICA approach. However, there has been no direct comparison of ICA and seed-based methods. Future study comparing different methods of analyzing rs-fMRI, including-but-not-limited-to seed-based and ICA approaches, may clarify the optimum approach for language lateralization using rs-fMRI.
We compared rs-fMRI with tb-fMRI as the reference standard, but there are limitations with tb-fMRI. A meta-analysis found that tb-fMRI correctly classified 94% of patients with epilepsy as having typical language lateralization based on the intracarotid amobarbital procedure, but only 51% of patients were correctly classified as having atypical language lateralization.31 Hence, tb-fMRI works well when patients have typical language lateralization but not when patients have atypical language lateralization. While it would have been ideal to compare rs-fMRI with the intracarotid amobarbital procedure (the criterion standard) or electrophysiologic mapping with intracranial electrodes, both procedures are performed only occasionally in clinical practice in children with DRE. Hence, we were unable to compare with these 2 methods in the current study. We considered the whole language network, but a portion of patients with epilepsy are known to demonstrate cross-dominant language laterality and, thus, discordant lateralization between expressive and receptive language regions. This finding could impact calculation of the LI and, therefore, concordance between rs-fMRI and tb-fMRI. The language network in the Willard atlas does not include secondary language areas such as the dorsolateral prefrontal cortex, presupplementary motor area, visual word form area, and basal temporal language area. Hence, these areas were not considered when assessing language laterality, which may potentially impact subsequent classification of hemispheric dominance. Future studies assessing language laterality with rs-fMRI should consider using a language template that incorporates secondary language areas. Finally, we did not consider the dynamic functional connectivity of rs-fMRI, which would have accounted for a time-varying language network connectivity and may impact LI assessment. Hence, both static and dynamic functional connectivity of language networks should be evaluated in future studies determining language lateralization.
CONCLUSIONS
The current study found only modest concordance of rs-fMRI with tb-fMRI in determining language lateralization in children with DRE. While accuracy rates were reasonably high when children were left-lateralized for language, rs-fMRI was poor at correctly lateralizing language in children who had atypical language dominance compared with tb-fMRI as the reference standard. As such, based on the findings of the current study, caution is recommended if using rs-fMRI to lateralize language function in children with DRE undergoing presurgical evaluation. Further studies comparing rs-fMRI with the intracarotid amobarbital procedure or electrophysiologic mapping, as well as addressing the limitations of the current study, are required to confirm the use of rs-fMRI in presurgical mapping of children with epilepsy.
Footnotes
The opinions, results, and conclusions are those of the authors, and no endorsement by the Ontario Brain Institute is intended or should be inferred.
This research was supported by EpLink, the Epilepsy Research Program of the Ontario Brain Institute. The Ontario Brain Institute is an independent nonprofit corporation, funded partially by the Ontario government.
Disclosures: Anwar S. Shatil—UNRELATED: Employment: The Hospital for Sick Children, Peter Gilgan Centre for Research and Learning. Cristina Go—UNRELATED: Employment: The Hospital for Sick Children, Division of Neurology. Elysa Widjaja—RELATED: Grant: Ontario Brain Institute.* *Money paid to the institution.
References
- Received September 25, 2020.
- Accepted after revision February 16, 2021.
- © 2021 by American Journal of Neuroradiology