Abstract
BACKGROUND AND PURPOSE: Patients with SAH due to a ruptured intracranial aneurysm occasionally show reversible high-signal lesions in the splenium of the corpus callosum on DWI. These lesions are called cytotoxic lesions of the corpus callosum. This study retrospectively reviewed cases of aneurysmal SAH and investigated clinical features of cytotoxic lesions of the corpus callosum associated with SAH.
MATERIALS AND METHODS: Participants comprised 259 patients with aneurysmal SAH who had undergone curative treatment at our hospital. We examined the following items related to cytotoxic lesions of the corpus callosum: occurrence rate, timing of appearance and disappearance of the lesions, lesion size, aneurysm location, severity of SAH, treatment method, clinical course, and outcome.
RESULTS: Among the 259 cases, DWI detected cytotoxic lesions of the corpus callosum in 33 patients (12.7%). The mean periods from the onset of SAH to detection and disappearance of cytotoxic lesions of the corpus callosum were 6.3 days (range, 0–25 days) and 35.7 days (range, 9–78 days), respectively. Cytotoxic lesions of the corpus callosum were classified into 2 types: a small type localized in the splenium in 26 cases (78.9%) and a large type spread along the ventricle in 7 cases (21.2%). The severity of SAH, coiling, hydrocephalus, and poor mRS score at discharge were significantly higher in the group with cytotoxic lesions of the corpus callosum. However, multivariate analysis did not identify cytotoxic lesions of the corpus callosum as a risk factor for poor outcome.
CONCLUSIONS: Cytotoxic lesions of the corpus callosum appear at a frequency of 12.7% in patients with aneurysmal SAH. Cytotoxic lesions of the corpus callosum associated with SAH take several days to appear and subsequently resolve within about a month. Cytotoxic lesions of the corpus callosum were likely to occur in patients with high-grade SAH but did not represent a predictor of poor outcome.
ABBREVIATIONS:
- CLOCC
- cytotoxic lesions of the corpus callosum
- DCI
- delayed cerebral ischemia
MR imaging findings of cytotoxic lesions of the corpus callosum (CLOCC) are known to appear in association with various pathologic conditions such as encephalitis, encephalopathy, metabolic disorder (hypoglycemia and hypernatremia), antiepileptic drug withdrawal, alcoholism, infection, and seizure.1⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓-14 A series of cases showing CLOCC associated with coronavirus disease 2019 was also recently reported.15 These lesions are clearly detectable, especially on DWI.13 In patients with SAH due to a ruptured intracranial aneurysm, reversible high-signal lesions (ie, CLOCC) are sometimes observed on DWI in the splenium of the corpus callosum during the clinical course, but the frequency of appearance and associations with clinical findings remain unclear.14,16 The present study retrospectively reviewed cases of aneurysmal SAH and investigated the clinical features of CLOCC associated with SAH.
MATERIALS AND METHODS
Patient Data
This study included 286 patients with aneurysmal SAH who had been treated in the Department of Neurosurgery at Kawasaki Medical School between April 2009 and June 2016. Any patients who did not undergo serial MR imaging or who had SAH of unknown origin were excluded. The management, treatment, and clinical course of patients with SAH that does not require clipping or coiling, such as in perimesencephalic SAH, differ markedly in patients with SAH requiring treatment.17 To minimize variability in the investigated cohort, we, therefore, included only patients with an aneurysm that was identified and subsequently clipped or coiled. Patients with traumatic SAH were also excluded. The main confounder for callosal lesions in patients with SAH having experienced trauma is diffuse axonal injury. We retrospectively examined patients with aneurysmal SAH focusing on CLOCC. Clinical data were collected from the medical records of patients, and we examined the following items related to the lesion: timing of the appearance and disappearance of lesions; locations of aneurysms; history of hypertension and diabetes mellitus; severity of SAH according to both Hunt and Hess grade and Fisher group; treatment method; clinical course involving development of delayed cerebral ischemia (DCI) and/or hydrocephalus; and mRS score at discharge.
“Clinical deterioration caused by DCI” is a concept proposed by a multidisciplinary research group as an important outcome measure in patients with aneurysmal subarachnoid hemorrhage.18 Briefly, clinical deterioration caused by DCI is a secondary outcome measure in patients with aneurysmal subarachnoid hemorrhage defined as the following: “The occurrence of focal neurologic impairment (such as hemiparesis, aphasia, apraxia, hemianopia, or neglect), or a decrease of at least two points on the Glasgow Coma Scale (either on the total score or on one of its individual components [eye, motor on either side, verbal]). This should last for at least 1 hour, is not apparent immediately after aneurysm occlusion, and cannot be attributed to other causes by means of clinical assessment, CT or MR imaging scanning of the brain, and appropriate laboratory studies.”18
Imaging Methods
In all cases, DWI, FLAIR, T2WI, and MRA were performed before and after clipping or coiling with 3T scanners (Vantage Titan 3T MRT-3010, Canon Medical Systems; or Ingenia 3T CX, Philips Healthcare) or 1.5T scanners (Excelart Vantage MRT-2003, Canon Medical Systems; or Signa Excite, GE Healthcare). Acquisition parameters for DWI were the following: TR, 6000 ms; TE, 95 ms; matrix, 256 × 128 or 192 × 128; FOV, 24 cm; section thickness, 5 mm; interslice gap, 0.5; axial sections, 26; flip/flop angle, 90°/180; b-values, 0 and 1000 s/mm2.
Patients underwent MR imaging at the time of admission, once in the first week, and once in the second week. In some cases, more images were obtained at the discretion of the attending physicians. We defined CLOCC as high-signal areas observable in the splenium of the corpus callosum on DWI that disappeared during follow-up. We chose DWI as the sequence to confirm the disappearance of CLOCC because of its clarity, though CLOCC can also be observed with FLAIR and T2WI. We also confirmed that CLOCC showed a reduced ADC corresponding with the DWI hyperintensity to exclude T2-shinethrough artifacts. CLOCC can be distinguished from ischemia because they also disappear on T2WI and FLAIR. We classified CLOCC into a small type localized to the splenium and a large type extending along the ventricle, in accordance with previous reports, and examined the ratio of types.9,16
Treatment of Aneurysms and General Management
All patients underwent digital subtraction angiography on admission. Selection of treatment with clipping or endovascular coil embolization resulted from a consensus reached between the treating neurosurgeon and the interventional neuroradiologist after analyzing the risks and chances of success of both therapeutic modalities for each case. All patients received 0.9% normal saline at a rate of 1 mL/kg/h, with an appropriate dosage of supplemental 5% albumin solution administered to maintain positive fluid balance. Oral statins and mineralocorticoids were not routinely administered. Persistent fever (temperature exceeding 38.5°C) was treated with acetaminophen and surface cooling devices. Angiography was routinely performed on patients in whom DCI developed, and endovascular treatment of vasospasm entailed either intra-arterial chemical vasodilation with fasudil hydrochloride hydrate (Eril; Asahi Kasei Pharma) or balloon angioplasty. We did not change the treatment protocol after detecting CLOCC. The protocol for this retrospective study was approved by the ethics committee at our institution, and the board waived the requirement to obtain patient consent (approval No. 2780).
Statistical Analysis
Numeric data are expressed as mean [SD] or median (interquartile range). Categoric variables are expressed as numbers (percentages). Statistical analyses were performed using SPSS statistical and computing software, Version 24 (IBM). For intergroup comparisons, we used the χ2 test, Fisher exact test, Student t test, and the Mann-Whitney U test. To assess independent predictors of poor outcome, we first performed univariate logistic regression; then, those possible predictors showing values of P < .10 were included in the multivariate analysis. In all analyses, values of P < .05 were considered statistically significant.
RESULTS
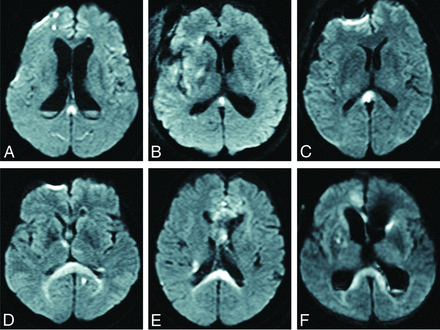
Of the 286 cases of aneurysmal SAH, we excluded 12 cases that could not be evaluated due to early death, 10 cases that showed SAH of unknown origin, and 5 cases in which only external ventricular drainage was performed. The remaining 259 patients underwent curative treatment (clipping or coiling) and MR imaging multiple times, and we analyzed these cases. Of the 259 cases, DWI detected CLOCC in 33 patients (12.7%). These patients comprised 5 men and 28 women, with a mean age of 68.6 years (range, 40–94 years). In 33 cases with CLOCC, the mean period from the onset of SAH to detection of CLOCC was 6.3 days (range, 0–25 days). MR imaging showed CLOCC in 4 cases on day zero, 21 cases within days 1–7, five cases within days 8–14, and 3 cases on or after day 15. CLOCC were detected after clipping or coiling in 25 cases (75.8%) and before clipping or coiling in 8 cases (24.2%). CLOCC resolved in all cases, and the mean period from onset of SAH to the disappearance of CLOCC was 35.7 days (range, 9–78 days). On DWI, CLOCC were classified as the small type localized to the splenium (mean maximum diameter, 10.4 mm) or the large type extending along the ventricle (Fig 1). Among all 33 patients with CLOCC, 26 patients (78.9%) showed the small type and 7 cases (21.2%) showed the large type. CLOCC were identified as asymmetric in 16 of the 33 patients.
Two types of CLOCC: a small type localized to the splenium (A–C) and a large type spreading along the ventricle (D–F).
Clinical characteristics of patients with and without CLOCC are shown in Table 1. Regarding age, severity of the Hunt and Hess grade, severity of the Fisher score group, and treatment method, significant differences were seen between groups. The CLOCC group was significantly younger, showed greater severity of SAH, and was more likely to undergo coil embolization than the group without CLOCC. No significant difference existed between groups regarding sex, history, or aneurysm location. Development of hydrocephalus and a poor mRS score at discharge were significantly more frequent in the group with CLOCC (Table 2), though no significant differences were seen between groups regarding DCI after SAH. In univariate analysis, advanced age, severity of the Hunt and Hess grade, large hematoma, coiling, and appearance of CLOCC correlated with poor outcome (Table 3). In multivariate analysis, only old age, severity of the Hunt and Hess grade, and large hematoma correlated with poor outcome (Table 3). Multivariate analysis, thus, did not confirm CLOCC as a risk factor for poor outcome.
Characteristics of patients with or without CLOCC
Clinical outcomes of patients with or without CLOCC
Uni- and multivariate analyses of variables associated with poor outcomea
Illustrative Case
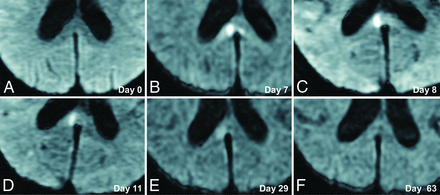
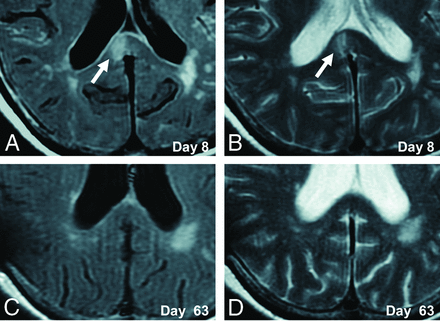
A 70-year-old woman presented with poor-grade SAH (Hunt and Hess grade 4). CT revealed Fisher group 3 SAH, and MRA demonstrated a 5-mm-diameter aneurysm on the anterior communicating artery. The patient underwent aneurysm coiling on day 2. This patient developed DCI and received regional arterial infusion of fasudil hydrochloride hydrate 3 times. She also developed hydrocephalus and underwent ventriculoperitoneal shunting. The patient had mild disorientation and was discharged home with an mRS score of 3. Although no abnormality was evident on DWI in this patient on admission (Fig 2A), a high-signal area appeared in the splenium of the corpus callosum on SAH day 7 (Fig 2B). The lesion gradually faded and had completely resolved by day 63 (Fig 2C–F). This change was thought to represent CLOCC and was also observable on FLAIR and T2WI (Fig 3).
Serial DWI in an illustrative case. A 70-year-old woman presented with poor-grade SAH due to rupture of an aneurysm (diameter, 5 mm) at the anterior communicating artery. DWI on admission shows no lesion (A). CLOCC appear on days 7–8 (B and C) and gradually resolve (D–F).
Serial FLAIR and T2WI in an illustrative case. CLOCC (white arrows) are observed in the splenium on FLAIR and T2WI on day 8 (A and B) and resolve by day 63 (C and D).
DISCUSSION
Clinical Features of CLOCC Associated with SAH
The splenium is known as a region with characteristics differing from those of other parts of the corpus callosum. The splenium consists of fibers of the forceps major and communicates somatosensory information between the left and right parietal lobes and the visual cortex at the occipital lobes. Nerve fibers in the splenium show specific features, with the density of thin fibers reportedly decreasing and the density of large-diameter fibers increasing.19 Various congenital and acquired diseases can involve the splenium of the corpus callosum. These include vascular diseases such as cerebral infarction and bleeding; demyelinating diseases such as multiple sclerosis and acute disseminated encephalomyelitis; brain tumors such as lipoma, glioma, and malignant lymphoma; traumatic lesions such as diffuse axonal injury; and encephalopathy caused by hypoxia, hypoglycemia, and carbon monoxide poisoning.
Starkey et al14 described CLOCC associated with SAH in 3 different cases that were nontraumatic and presumably aneurysmal, and in 1 case involving aneurysmal dissection. The Japanese literature includes a report by Yamazaki et al16 of 7 cases of CLOCC associated with SAH. Our study is by far the largest to date. Uchida et al20 also reported a case of ruptured arteriovenous malformation with CLOCC in the Japanese literature. Sorimachi et al21 reported hemorrhage in the splenium of the corpus callosum in association with SAH, but that change was deemed unrelated to CLOCC. Hadeishi et al22 reported that DWI findings at the onset of SAH were related to the initial brain injury, but they mainly discussed cortical lesions and did not mention splenial lesions.
The most important finding in the present study was that CLOCC, which have been described in relation to various diseases and conditions, were also observed in cases of aneurysmal SAH and that the frequency of the lesions was found to be 12.7% among treated patients with SAH. The possibility remains that CLOCC are associated with elevated intracranial pressure because CLOCC appeared more frequently among patients with higher Hunt and Hess grades. Although no direct association between intracranial hypertension and CLOCC has been reported, intracranial hypertension is associated with strong activation of the inflammatory cascade, resulting in high cytokine levels in the brain.23 Increased intracranial pressure is thus speculated to represent 1 cause of CLOCC. This possibility is not limited to CLOCC in cases of SAH rather than in CLOCC of other etiologies, but increased cytokine levels might be more prominent in severe cases of SAH because of the extremely high intracranial pressure. The high incidence of CLOCC among patients who underwent coil embolization may be related to the tendency to choose coiling in poor-grade cases. Hydrocephalus usually becomes apparent around 7–10 days after SAH and is most prominent around 3–4 weeks, except for acute hydrocephalus appearing immediately after severe SAH.24 Hydrocephalus usually shows normal pressure and is not associated with high intracranial pressure in the early stages of SAH.
Frequencies of poor mRS scores at discharge were higher in the CLOCC group, but multivariate analysis failed to identify CLOCC as a predictor of poor outcome. Un-necessary examinations and treatments thus appear avoidable even if this lesion is identified on MR imaging. Neurosurgeons do not need to perform repeat angiography or MR imaging and do not need to initiate treatment for encephalitis or encephalopathy.
Regarding lesion size, Takanashi et al9 classified CLOCC into 2 types: one isolated within the splenium and the other involving the entire corpus callosum. We therefore divided the CLOCC into a small type (isolated within the corpus callosum) and a large type (extended along the ventricle). Yamazaki et al16 reported 6 oval-type cases and 1 extended-type case among their 7 cases with CLOCC. Their oval-type cases were presumed to correspond to our small-type cases, while their extended-type cases corresponded to our large-type cases, though lesion size was not considered.16 No reports have clarified the reasons for asymmetric CLOCC. At present, the implications of CLOCC size and shape remain obscure.9,16
What Are CLOCC?
We speculated that CLOCC do not represent the initial brain injury due to SAH and should, instead, be considered a secondary change following SAH. First, CLOCC were detected within about a week after SAH onset and disappeared within around 1 month. Second, this MR imaging finding is observable in a wide variety of other diseases and conditions. CLOCC can be distinguished from ischemia because they also resolved on T2WI and FLAIR. CLOCC do not represent ordinary ischemia and seem related to some abnormality of the cerebral circulation and metabolism associated with SAH, considering that the lesion also resolved on T2WI and FLAIR. Liu and Lin25 mentioned transient intramyelinic edema as a possible mechanism for CLOCC. The neuroradiologic finding of transient, isolated, high-intensity signal on DWI involving the lesion might indicate cytotoxic edema. Specifically, intramyelinic edema is a subtype of cytotoxic edema involving the myelin sheaths that was postulated by Liu and Lin25 to be a possible mechanism underlying CLOCC.26
Why CLOCC exclusively localize to the splenium remains unclear. We speculate on the mechanism of CLOCC associated with SAH as follows:14 SAH causes increases in levels of cytokine-like interleukin-6 and tumor necrosis factor-α in CSF;27,28 astrocytes might then be stimulated by cytokines to release glutamate and block glutamate reuptake, increasing extracellular glutamate levels. Ultimately, this cascade would lead to dysfunction of the neurons and microglia, with cytotoxic edema developing as water becomes trapped in these cells. The splenium of the corpus callosum is vulnerable to cytokinopathy because astrocytes and oligodendrocytes of the splenium have a higher density of receptors for cytokines and glutamate compared with those in other brain areas. This higher density would lead to a tendency for cytotoxic edema of the splenium, manifesting as CLOCC on DWI. Another hypothesis is based on the blood flow to the splenium. The splenium is supplied not only by the anterior circulation but also by the posterior circulation and so has more abundant blood flow than other areas of the corpus callosum supplied solely by the anterior circulation.29⇓-31 The splenium may therefore be more susceptible to cytotoxic edema caused by cytokine release in the blood compared with other brain areas.
All patients received intravenous fasudil hydrochloride hydrate every 8 hours from day 4 to day 14. We administered fasudil to prevent cerebral vasospasm. Basic research has shown that intravenous administration of fasudil dose-dependently increased regional cerebral blood flow and blood velocity, without changing blood volume.32 On the basis of these results, fasudil can be inferred to not increase the cytokine exposure of the splenium. The present report is a study of patients with SAH who received standard treatment in Japan, although it cannot be completely ruled out that intravenous administration of fasdil may have affected the appearance of CLOCC.
The relevance of CLOCC to clinical symptoms is difficult to determine because clinical symptoms did not change when CLOCC appeared. Physicians should be aware that CLOCC occur frequently with SAH and are not a cause for panic, because this finding is not a predictor of poor outcome. However, the chronic effects of CLOCC are unknown, and careful follow-up is needed.
The present study has several limitations that warrant consideration. First, the sample size was not exceptionally large, and larger-scale research is warranted. However, this was the first report to investigate the incidence of CLOCC in patients with SAH and thus provides a contribution to future studies. Second, this was a retrospective study, and more systematic research is needed in the future. Third, we could not obtain any pathologic specimens for CLOCC. To solve the mystery of CLOCC, pathologic study and further accumulation of data are needed.
CONCLUSIONS
CLOCC occurred in SAH at a frequency of 12.7%. CLOCC associated with SAH developed in the acute phase, took several days to appear, and subsequently resolved within about a month. CLOCC were more likely to occur in high-grade cases of SAH but were not confirmed as a predictor of poor outcome. CLOCC represent a conspicuous imaging finding in treated patients with aneurysmal SAH, but their clinical importance should not be overemphasized.
ACKNOWLEDGMENTS
We appreciate the assistance of Yukari Ogawa, Keita Kinoshita, Satoshi Hirai, and Hiroki Takai (Department of Neurosurgery, Kawasaki Medical School, Kurashiki, Okayama, Japan) in the acquisition of data.
References
- Received August 9, 2020.
- Accepted after revision December 1, 2020.
- © 2021 by American Journal of Neuroradiology