Abstract
BACKGROUND AND PURPOSE: Currently, the characteristics of carotid plaques are considered important factors for identifying subjects at high risk of stroke. This study aimed to test the hypothesis that carotid plaque composition assessed by CTA is associated with an increased risk of future major adverse cardiovascular events among asymptomatic subjects with moderate-to-severe carotid artery stenosis.
MATERIALS AND METHODS: This single-center, retrospective cohort study included 194 carotid plaques from 176 asymptomatic subjects with moderate-to-severe carotid artery stenosis. The association of CTA-determined plaque composition with the risk of subsequent adverse cardiovascular events was analyzed.
RESULTS: During a median follow-up of 41 months, the adverse cardiovascular event incidence among 194 carotid plaques was 19.6%. There were significant differences in plaque Hounsfield units (P < .001) and spotty calcium presence (P < .001) between carotid plaques from subjects with and without subsequent adverse cardiovascular events. Multivariable analysis revealed carotid plaque Hounsfield unit density (P < .001) and spotty calcium (P < .001) as independent predictors of subsequent adverse cardiovascular events. In association with moderate carotid artery stenosis, the plaque Hounsfield unit values were significantly lower among carotid plaques from subjects who experienced subsequent adverse cardiovascular events (P = .002), strokes (P = .01), and cardiovascular deaths (P = .04); the presence of spotty calcium was significantly associated with the occurrence of adverse cardiovascular events (P = .001), acute coronary syndrome (P = .01), and cardiovascular death (P = .04).
CONCLUSIONS: Carotid plaque Hounsfield unit density and spotty calcium were independent predictors of a greater risk of adverse cardiovascular event occurrence.
ABBREVIATIONS:
- ACS
- acute coronary syndrome
- CAS
- carotid artery stenosis
- DUS
- Doppler ultrasound
- HR
- hazard ratio
- IQR
- interquartile range
- MACE
- major adverse cardiovascular event
Cardiovascular disease results in considerable morbidity and mortality worldwide; therefore, the identification of subclinical disease during the asymptomatic phase is an important public health goal.1 Carotid atherosclerosis has been assessed by Doppler sonography (DUS)-determined intima-media thickness or plaque features for predicting cardiovascular disease, particularly cerebrovascular disease.2 Therefore, carotid DUS screening could be a valuable tool for assisting with cardiovascular risk stratification to inform preventive strategies for asymptomatic subjects with few conventional risk factors.3 However, DUS can be limited by operator-dependent variability, low image resolution, and acoustic shadowing;4 therefore, the use of medical imaging to predict future cardiovascular or cerebrovascular events has been extensively investigated.5
Currently, a widely accepted concept is that carotid plaques go through a remodeling process, and sometimes atherosclerosis with even low-grade carotid artery stenosis (CAS) may result in cerebrovascular events.6 Thus, plaque characteristics other than the degree of stenosis alone may be important for identifying subjects at high risk of stroke.7 CTA provides simultaneous information about the degree of stenosis and a more detailed analysis of plaque composition.7 Coronary plaque characterization by CTA is a well-described method for predicting major adverse cardiovascular events (MACEs) in different subpopulations,8 whereas relatively little experience has been gained with CTA for identifying and quantifying carotid plaque composition associated with MACE risk.7 Therefore, this study aimed to test the hypothesis that carotid plaque composition detected by CTA can be used as a surrogate marker for predicting a higher risk of MACEs.
MATERIALS AND METHODS
Study Design and Study Sample
In this single-center, retrospective cohort study, we analyzed data extracted from the medical records of the subjects older than 50 years of age who underwent CTA for health screening or regular follow-up at our hospital. An electronic search was conducted using the hospital’s database to identify terms, including “carotid” and “stenosis,” on CTA reports.
Between January 2010 and December 2017, eight hundred seventy-six stenotic carotid arteries (876/4294, 20.4%) from 2147 consecutive CTAs were included in this analysis. The degree of CAS was measured according to the NASCET CTA criteria9 by an expert neuroradiologist. The exclusion criteria were as follows: 1) mild stenosis (<50% diameter reduction) or total occlusion (n = 422); 2) CAS with totally calcified plaque formation (n = 172); 3) diffuse stenosis, thin plaque, or artifacts precluding interpretation (n = 15); 4) previous ipsilateral carotid endarterectomy or stent placement (n = 12); and 5) recent cardiovascular events within 6 months (n = 61). Subtotal occlusions (n = 4) were included in this study. A total of 194 carotid plaques from 176 asymptomatic subjects without cardiovascular events within 6 months, with reported CAS in the range of 50% to 99% on baseline CTA and additional DUS, were included in the final analysis (Online Supplemental Data): moderate CAS (50%–69% diameter reduction, n = 101, 52.1%) and severe CAS (70%–99% diameter reduction, n = 93, 47.9%). Clinical indications for CTA were imaging surveillance for known cerebrovascular disease (including intracranial aneurysm or stenosis) and CAS (82%), and neurologic symptoms (including headache and change of mental status) (18%).
The demographics, risk factors of interest, clinical characteristics, and outcomes for all consecutive subjects were recorded in an Excel (Microsoft) database and analyzed retrospectively. Risk factor variables were defined as previously described.3 CTA reports were recorded by dedicated, board-certified neuroradiologists, and CTA images were independently re-evaluated for carotid plaque characterization, including the degree of CAS, plaque Hounsfield unit density, and the presence of ulceration, spotty calcium, napkin-ring sign, and calcium score by 2 specialized vascular surgeons and 1 neuroradiologist. All medication adjustments were made by the subjects’ health care providers at our hospital according to each individual’s atherosclerosis risk factors, determined using the American College of Cardiology Foundation/American Heart Association guidelines.9 Eligible carotid plaques from included subjects were followed from the date that they underwent CTA and were stratified into 2 groups according to the occurrence of MACEs during follow-up: carotid plaques with MACEs and carotid plaques without MACEs. Then, we performed a subgroup analysis of carotid plaques with moderate CAS.
Study Protocol Approvals, Registrations, and Patient Consent
Approval for data collection and publication was obtained from the institutional review board at our hospital (IRB No. 2019–0663), which waived the requirements for written informed consent because of the study’s retrospective design. All methods were performed in accordance with the relevant guidelines and regulations.
CTA Protocol
CTA was performed using a standard protocol (Online Supplemental Data) with the following parameters: 120 kV, automated tube current modulation using a 300-mA reference value, and a 1.0-mm reconstructed section thickness (Somatom Definition AS+ and Edge; Siemens). The scans were conducted from the aortic root to 3 cm above the skull before and after contrast injection. All CTA images were reconstructed in axial, coronal, and sagittal orientations.
Carotid Plaque Characterization
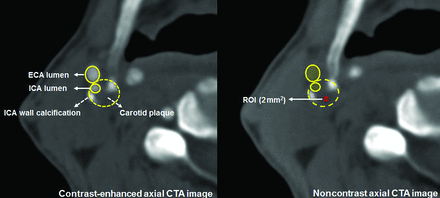
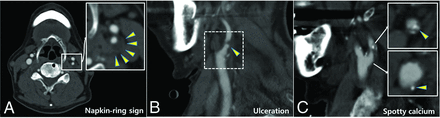
Each plaque was categorized according to the CTA findings as follows: calcified plaque composed predominantly of calcified tissue (>130 HU) (excluded from our analysis), mixed plaque composed of both calcified and noncalcified tissue, and soft plaque composed predominantly of noncalcified tissue. Carotid plaque ulceration was defined as the presence of an obvious, large excavation (>2 mm in depth) on the surface of the plaque.10 For Hounsfield unit measurements, an ROI of 2 mm2 was preselected on the visually least attenuated area of the plaque at the most stenotic level using a width of 850 HU and a level of 300 HU as the window-level settings on contrast-enhanced axial images to distinguish plaque from the vascular lumen. The plaque Hounsfield unit density was measured 5 times from the noncontrast axial images using prepositioned ROIs on the plaque to exclude blooming artifacts (Fig 1). The lowest measured Hounsfield unit value in each plaque was recorded. Calcium score, along with the presence of spotty calcium and the napkin-ring sign—suggested as high-risk plaque features for predicting acute coronary syndrome (ACS)—were also evaluated.11,12 Spotty calcium was defined as the presence of calcium in the plaque with a diameter of <3 mm in any direction on curved multiplanar reformation images, occupying only 1 side and a maximum arc below 90° on cross-sectional images.11,13 The napkin-ring sign was defined as the presence of low-attenuation plaque surrounded by a ring of high attenuation that was not >130 HU (Fig 2).12 The calcium score was measured using semiautomated software (Syngo CaScoring; Siemens) and the method described by Agatston et al.14
Representative figure of plaque Hounsfield unit value measurement. The plaque Hounsfield unit values were measured from noncontrast axial CTA images. An ROI of 2 mm2 was preselected on the visually least attenuated area of the plaque at the most stenotic level of contrast-enhanced axial images. Measurements were performed 5 times from the noncontrast axial images using prepositioned ROIs on the plaque. The lowest Hounsfield unit value in each plaque was recorded. ECA indicates external carotid artery.
Representative figures of high-risk features of carotid plaque (arrowheads) on CTA. Napkin-ring sign (A), carotid plaque ulceration (B), and spotty calcium (C).
Clinical Outcomes
The study outcomes included the occurrence of MACEs, defined as fatal or nonfatal, ipsilateral or contralateral stroke or TIA, ACS, or cardiovascular death. Data on all MACEs were centrally reviewed and blindly adjudicated by an experienced independent neurologist or cardiologist. Events were categorized as “Event,” “Limited data,” or “No Event” on the basis of chart review. Once an event was not confirmed due to Limited data, more information was collected by face-to-face or direct telephone interviews with the subjects or their families. For quality control, second reviews were conducted by other physicians blinded to the original adjudicated results. In the analysis, we included only ischemic strokes, as previously defined.3 ACS was defined as acute myocardial infarction or unstable angina pectoris, according to the American College of Cardiology/American Heart Association guidelines.15 Only the first event of each outcome was included in the MACE analysis, whereas each MACE was analyzed individually.
Statistical Analysis
Statistical analysis was performed using PASW Statistics for Windows, Version 18 (https://www.malavida.com/en/soft/pasw/); R, Version 3.6.3 (http://www.r-project.org); and SAS version 9.4 (SAS Institute). We separately summarized the characteristics of 176 subjects and 194 carotid plaques. Categoric variables are reported as frequencies or percentages, and continuous variables, as means (SDs) or medians and interquartile ranges (IQRs), as appropriate. Cox proportional hazard models, with robust standard errors that accounted for the clustering of subjects’ effects, were fitted to identify MACE predictors. The Mann-Whitney rank test was used for comparisons of non-normally distributed continuous variables. Demographic and clinical characteristics that were considered to be associated with MACEs and features of carotid plaques on CTA were included in this analysis. Variables yielding P values < .1 from univariable analysis were subjected to multivariable analysis, and hazard ratios (HRs) with 95% CIs were calculated. In the Kaplan-Meier survival analysis, Hounsfield unit values were dichotomized at the median value of the study cohort, and the log-rank test was used to compare the occurrence of MACEs and the individual MACE components. We performed subgroup analyses limited to carotid plaques from subjects with moderate CAS, wherein we evaluated the association between plaque composition and future MACE occurrences overall as well as the individual MACE components. The interobserver reproducibility of plaque characterization was analyzed through interclass correlation coefficient calculations. Possible interclass correlation coefficients ranged from 0 to 1.00, and measurement reliability was classified as excellent (interclass correlation coefficient > 0.9), good (0.75–0.9), moderate (interclass correlation coefficient = 0.5–0.75), or poor (interclass correlation coefficient < 0.5).16 P values < .05 were considered statistically significant.
RESULTS
After all exclusions based on the predetermined criteria, a total of 194 consecutive carotid plaques from 176 subjects were analyzed. All subjects were diagnosed with moderate-to-severe CAS using CTA and additional DUS. Eligible carotid plaques were stratified into 2 groups according to the occurrence of MACEs as follows: non-MACE group (156 plaques from 142 subjects, 80.4%) and MACE group (38 plaques from 34 subjects, 19.6%). Tables 1 and 2 show the subjects’ baseline, clinical, and plaque characteristics. The 2 groups did not differ significantly in terms of demographic characteristics, risk factors, or clinical characteristics, except that subjects in the MACE group were more likely to have atrial fibrillation. There were significant differences in plaque Hounsfield unit values (39.2 [SD, 15.2] versus 27.6 [SD, 12.7], P < .001) and spotty calcium presence (14.7% versus 44.7%, P < .001) between the 2 groups. The mean calcium score was higher in the MACE group than in the non-MACE group with a nonsignificant trend (P = .06), and the degree of CAS was not significantly different between the 2 groups (Online Supplemental Data).
Baseline and clinical characteristics of the study sample, stratified by the occurrence of MACEsa
Plaque characteristics, stratified by the occurrence of MACEsa
Variables Associated with MACE Occurrence
During follow-up, the MACE incidence among the 194 carotid plaques was 19.6% (38/194). There were 19 strokes (9.8%), 16 ACS diagnoses (8.2%), and 12 cardiovascular deaths (6.2%). According to the per-subject analysis (n = 176), there were 16 strokes (9.1%), 14 ACS diagnoses (8.0%), and 12 cardiovascular deaths (6.8%). Table 3 shows the results of the regression analysis in terms of MACE occurrences on a per-carotid plaque basis. In the adjusted models, plaque Hounsfield unit density (HR = 0.96; 95% CI, 0.94–0.98; P < .001) and spotty calcium presence (HR = 3.97; 95% CI, 2.08–7.60; P < .001) were independent predictors of future MACEs.
Cox proportional hazards model for variables associated with MACEa occurrence
Analysis of MACEs and Individual MACE Components Based on the Values Reflecting Plaque Hounsfield Unit Density and Spotty Calcium Presence
Cox proportional hazards modeling indicated a lower Hounsfield unit density among carotid plaques from subjects who experienced MACEs (HR = 0.96; 95% CI, 0.95–0.98; P < .001), whereas plaque Hounsfield unit density was not associated with all-cause mortality (P = .14) (Table 4). The prevalence of spotty calcium observed on CTA was higher among carotid plaques from subjects who experienced any MACE (HR = 4.08; 95% CI, 2.13–7.83; P < .001) when the composite outcome was analyzed as a single entity. When the individual MACE manifestations were analyzed separately, ipsilateral stroke (P = .09) and cardiovascular mortality (P = .06) were not associated with a higher prevalence of spotty calcium (Table 5).
Cox proportional hazards model for the association of the plaque Hounsfield unit values with the occurrence of the MACE composite outcome and the individual MACE components (n = 194)a
Cox proportional hazards model for the association of the presence of spotty calcium on CTA with the occurrence of the MACE composite outcome and the individual MACE components (n = 194)a
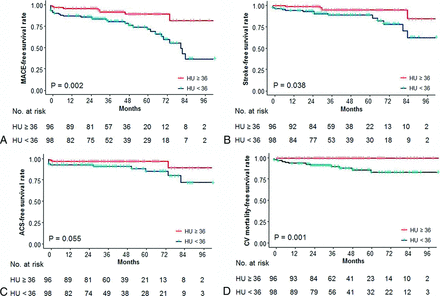
On Kaplan-Meier survival analyses of the cumulative event-free rates based on the dichotomization of Hounsfield units at the median value of the cohort (36 HU), a lower plaque density (<36 HU) was associated with decreased MACE-free (P = .002), stroke-free (P = .038), and cardiovascular mortality-free (P = .001) survival rates, compared with a higher plaque density (≥36 HU) (Fig 3). Although lower plaque density trended toward an association with a decreased ACS-free survival rate (P = .055), this was not statistically significant.
Kaplan-Meier survival analyses of the cumulative event-free rates based on the dichotomization of Hounsfield units at the median value of the cohort (36 HU). Cumulative event-free rates of MACE (A), stroke (B), ACS (C), and CV mortality (D), according to the carotid plaque density. CV indicates cardiovascular.
Subgroup Analysis of the MACE Composite Outcome and the Individual MACE Components among Carotid Plaques from Subjects with Moderate CAS Based on the Plaque Hounsfield Unit Density and Spotty Calcium Presence
The plaque Hounsfield unit values were lower among carotid plaques from subjects who experienced any MACE (HR = 0.95; 95% CI, 0.92–0.98; P = .002) and stroke (P = .01) and those who died from a cardiovascular cause (P = .04). However, ACS (P = .14) and all-cause death (P = .31) were not associated with plaque Hounsfield unit density (Online Supplemental Data). The prevalence of spotty calcium on CTA was higher among carotid plaques from subjects who experienced any MACE (HR = 5.20; 95% CI, 1.94–13.92; P = .001), ACS (P = .01), and cardiovascular death (P = .04). However, stroke (P = .07) was not associated with the presence of spotty calcium on CTA when analyzed individually (Online Supplemental Data). The low number of MACEs (n = 18) limited our ability to include additional variables in the multivariable models.
Interobserver Reliability
Good agreement between observers (2 vascular surgeons) was found in terms of the quantification of carotid plaque density (Hounsfield unit measurement) (interclass correlation coefficient = 0.831; 95% CI, 0.781–0.870) and spotty calcium presence (interclass correlation coefficient = 0.800; 95% CI, 0.734–0.849). There was also strong interobserver reliability between the vascular surgeon and the neuroradiologist for both Hounsfield units (interclass correlation coefficient = 0.862; 95% CI, 0.764–0.913) and spotty calcium (interclass correlation coefficient = 0.830; 95% CI, 0.731–0.931).
DISCUSSION
Carotid atherosclerosis is assessed by DUS-determined intima-media thickness or the degree of CAS for predicting cardiovascular disease, particularly cerebrovascular disease.2 However, in recent years, it has been shown that both carotid plaque composition and morphology could be important additional features in the risk assessment of patients with carotid artery atherosclerosis.10 In recent prospective clinical studies for imaging stroke biomarkers, high-risk carotid plaque features appeared to be associated with stroke in patients with moderate CAS.17,18 Atherosclerosis is a systemic disease, and the presence of atherosclerosis in a particular vascular bed is frequently associated with disease in other vascular territories. On the basis of prior studies,19,20 the correlation between carotid and coronary atherosclerosis is well-established. Lombardo et al20 found that patients with unstable plaques in the coronary arteries also tended to have unstable plaques in the carotid arteries; they posited that inflammation throughout the vasculature accounted for this finding. Recently, Brunner et al21 found that the detection of lipid cores on carotid MR imaging is associated with cardiovascular events. Furthermore, unstable carotid plaques seem to affect not only ipsilateral but also contralateral cerebral symptoms. Therefore, carotid plaques could be a surrogate marker acknowledging the systematic nature of the atherosclerotic processes affecting different vascular beds.22
Recent studies support the concept that carotid plaques go through remodeling;6 some plaques regress and stabilize to be less likely to result in clinical events, whereas some plaques progress to high-risk, unstable plaques with large lipid necrotic cores and thin fibrous caps, which can rupture and cause clinical events.23⇓⇓-26 The stability of atherosclerotic plaques largely depends on the composition and morphology of the plaques. Thus, plaque characteristics other than the degree of stenosis alone are important for identifying subjects at higher risk of clinical events, not only for decisions about medical therapy but also for decisions about aggressive interventions.5⇓-7 In this regard, the use of noninvasive imaging modalities to detect high-risk plaques and predict their natural evolution has been extensively investigated,7,27 and several different imaging modalities, such as CTA, MR imaging, DUS, and PET have been used to assess plaque stability. The advantage of DUS lies in the detection of initial wall alterations, whereas MR imaging can characterize various plaque components. CTA has basically been used to quantify plaque subcomponents, including fatty, mixed, or calcified constituents. Although CTA is known to be less effective than MR imaging for detecting intraplaque hemorrhage or fibrous cap status, Ajduk et al24 found multidetector row CTA to be highly sensitive and moderately specific for detecting intraplaque hemorrhage.24,28
CTA provides simultaneous information about the degree of luminal stenosis, a more detailed analysis of plaque composition,7 and high-resolution 3D plaque imaging in a relatively short time. These features make this method particularly suitable for its use in clinical practice.27 Coronary plaque characterization by CTA is well-established for predicting MACEs in different subpopulations, with higher reproducibility than other imaging modalities.8 Although many studies have been performed to differentiate carotid plaque compositions and to compare unstable with stable plaques using histologic analysis through Hounsfield unit measurements on CTA,10,27 few studies have independently focused on the CTA characteristics of different carotid plaques in association with future cardiovascular event risks.
In this study, we restricted our analysis to the 4 plaque characteristics commonly associated with ischemic complications.29 Although spotty calcium detected via coronary CTA has been reported to be an important prognostic factor for ACS,30 the association of spotty calcium in carotid CTA with MACEs has been rarely investigated.31 Spotty calcium is likely to exist in lipid-rich plaques and be associated with inflammatory processes and vulnerability, unlike large calcifications in fibrocalcific plaques.13,32 Hounsfield unit values are known to reflect plaque composition; lower Hounsfield unit values are associated with higher probabilities of unstable plaques with large lipid cores and intraplaque hemorrhage.25,33 It is well accepted that the napkin-ring sign and higher calcium scores in coronary CTA are closely associated with MACEs.29,33 Although the napkin-ring sign and calcium score in the carotid plaques were not associated with MACEs in our analysis, we found that plaque Hounsfield unit values and spotty calcium presence were independent predictors of future MACEs, and these could be surrogate markers of the atherosclerotic processes affecting different vascular beds.
The strength of this study was its design, which was suitable for investigating the association between carotid plaque composition and the risk of MACEs among asymptomatic subjects with CAS. Previous CT-based studies have been cross-sectional, comparing the composition of stable and unstable carotid plaques or symptomatic and asymptomatic plaques.34 Carotid atherosclerosis is a complicated, dynamic process, which persists across time, even in cases of asymptomatic mild CAS. Through a remodeling process, carotid plaques may also stabilize, leading to a phenotype reversal from unstable to stable. Whereas the association between plaque composition and MACE risk has long been suspected, confirmation of this hypothesis requires reliable plaque assessments at baseline, followed by longitudinal assessments of clinical outcomes.34 Cross-sectional studies cannot evaluate chronologic plaque changes; this issue may result in some differences in the assessments of plaque composition.
This study had important limitations that should be acknowledged. First, this was a retrospective analysis subject to selection bias. A number of subjects were excluded, and some clinical information was not available from the medical records. Calcified plaques (>130 HU), mild stenosis (<50%), and plaques with small volumes precluding interpretation were excluded from our analysis due to measuring difficulties. The presence of calcium artifacts is one of the main limitations of CTA because calcifications may obfuscate the correct quantification of the lipid core. Although the role of severe calcification in carotid plaque evaluation still needs to be clarified, several studies have postulated that a high degree of plaque calcification may be considered a protective factor, especially when the tissue is located superficially.27 Despite the controversy,35,36 higher carotid calcium scores seem to be associated with higher MACE risk.33 In our analysis, the mean calcium score was higher in the MACE group than in the non-MACE group, with a nonsignificant trend. However, higher carotid calcium scores were not associated with higher MACE risk.
Second, plaque Hounsfield unit density was measured via manual positioning of the ROIs. Although there was good agreement among observers in our analysis, manual ROI placement is time-consuming and may be subject to operator bias and beam-hardening artifacts from surrounding tissue. Previous studies have suggested various techniques for selecting ROIs to measure carotid plaque Hounsfield unit values.24,37 Clinical feasibility and reproducibility are important for carotid plaque analyses. Manual selection of larger ROIs is difficult for irregularly shaped or circumferential carotid plaques,38 which are more at risk of being affected by adjacent tissue densities, and manual selection is more operator-dependent. Although manual ROI positioning may be inferior to automatic or semiautomatic ROI measurement using plaque-analysis software,27,34,39 it is advantageous over measurement using plaque-analysis software in terms of feasibility and widespread availability.28 Third, we did not assess plaque volume as an independent predictor of MACEs. Fourth, the plaque Hounsfield unit density did not fully reflect the density of the entire plaque because we measured the Hounsfield unit values at minimally attenuated areas of the plaques at the most stenotic level. Finally, as with all observational studies, we cannot draw conclusions about causality, and our results should be considered as hypothesis-generating rather than definitive.
CONCLUSIONS
Despite all of these limitations and the debate regarding plaque interpretation by CTA, this pilot study provided evidence of an association between the state of carotid plaque composition, as evaluated by CTA, and the future risk of MACEs among subjects with asymptomatic CAS. The lower the carotid plaque Hounsfield unit density was, the higher was the probability that the artery was associated with the subsequent occurrence of a MACE; spotty calcium in the carotid plaque was an independent and significant predictor of greater subsequent MACE risk. The determination of plaque composition by CTA can provide valuable information for predicting the natural behavior of carotid plaques causing moderate to severe stenosis.
References
- Received November 8, 2020.
- Accepted after revision July 28, 2021.
- © 2021 by American Journal of Neuroradiology