Abstract
BACKGROUND AND PURPOSE: Thrombus embolization during mechanical thrombectomy occurs in up to 9% of cases, making secondary medium vessel occlusions of particular interest to neurointerventionalists. We sought to gain insight into the current endovascular treatment approaches for secondary medium vessel occlusion stroke in an international case-based survey because there are currently no clear recommendations for endovascular treatment in these patients.
MATERIALS AND METHODS: Survey participants were presented with 3 cases involving secondary medium vessel occlusions, each consisting of 3 case vignettes with changes in the patient’s neurologic status (improvement, no change, unable to assess). Multivariable logistic regression analyses clustered by the respondent’s identity were used to assess factors influencing the decision to treat.
RESULTS: In total, 366 physicians (56 women, 308 men, 2 undisclosed) from 44 countries provided 3294 responses to 9 scenarios. Most (54.1%, 1782/3294) were in favor of endovascular treatment. Participants were more likely to treat occlusions in the anterior M2/3 (74.3%; risk ratio = 2.62; 95% CI, 2.27–3.03) or A3 (59.7%; risk ratio = 2.11; 95% CI, 1.83–2.42) segment compared with the M3/4 segment (28.3%; reference). Physicians were less likely to pursue endovascular treatment in patients who showed neurologic improvement than in patients with an unchanged neurologic deficit (49.9% versus 57.0% responses in favor of endovascular treatment, respectively; risk ratio = 0.88, 95% CI, 0.83–0.92). Interventionalists and more experienced physicians were more likely to treat secondary medium vessel occlusions.
CONCLUSIONS: Physicians’ willingness to treat secondary medium vessel occlusions endovascularly is limited and varies per occlusion location and change in neurologic status. More evidence on the safety and efficacy of endovascular treatment for secondary medium vessel occlusion stroke is needed.
ABBREVIATIONS:
- EVT
- endovascular treatment
- LVO
- large-vessel occlusion
- MeVO
- medium vessel occlusion
- RR
- risk ratio
Medium vessel occlusions (MeVOs) (ie, occlusions of the M2, M3, A2, A3, P2, or P3 vessel segment) account for 25%–40% of all acute ischemic stroke cases.1,2 They can be classified as primary and those that occur de novo (similar to large-vessel occlusion, [LVO]) or secondary and those that occur due to breakdown of LVO and migrate into more distal vessel segments.
Secondary MeVOs have been shown to occur in up to 14% of patients with LVO without treatment or intervention due to spontaneous thrombus migration and fragmentation.3 Iatrogenic secondary MeVOs can be induced either by thrombolytic treatment or endovascular treatment (EVT).4,5 With periprocedural embolization occurring in up to 9% of all EVT cases,4 secondary MeVOs are of particular interest to neurointerventionalists.
The clinical course of MeVO strokes can be poor if left untreated. In the case of secondary MeVO, one would expect even worse outcomes because the affected area will be larger (ie, from the initial LVO) than in primary MeVOs.1 In the setting of rapid material and technique developments enabling improved EVT efficacy and safety, EVT indications could likely be expanded to more distal vessel occlusions. Due to a lack of data from randomized trials, however, there is currently neither reliable evidence nor expert consensus on whether EVT is safe and effective for MeVO strokes. Moreover, secondary MeVOs occurring before or during EVT are often only discovered on the first or control DSA runs. In such cases, the procedure has already begun, so the question remains whether to continue with EVT and not whether it is indicated. We sought to gain insight into the current management approaches regarding EVT in acute ischemic stroke cases caused by secondary MeVOs using a Web-based survey with prespecified case scenarios.
MATERIALS AND METHODS
Survey Design
To better understand current treatment practices and endovascular decision-making in cases of acute ischemic stroke caused by MeVO, we conducted an international online, cross-sectional, anonymous, invitation-only survey (MeVO-Finding Rationales and Objectifying New Targets for IntervEntional Revascularization in Stroke; MeVO-FRONTIERS) using Qualtrics (www.qualtrics.com) among stroke physicians. The survey took approximately 30 minutes to complete. Response data were obtained from November 12, 2020, to December 31, 2020. Approval by Conjoint Health Research Ethics Board of the University of Calgary was obtained. Data used in the current study are available from the author on reasonable request.
Survey Participants
Approximately 1400 stroke physicians (neurologists, interventional neurologists, interventional neuroradiologists, interventional radiologists, neurosurgeons, and other physicians directly involved in acute stroke care) were invited to participate in this survey through personal and professional networks of the study authors. No restrictions with regard to case volume or experience level were applied, and participants were from both academic and nonacademic backgrounds. Before accessing the case scenarios, the physicians provided basic personal data (age range, sex, years of experience in stroke treatment, annual personal stroke treatment volume, annual center stroke treatment volume, geographic region, subspecialty, and teaching-versus-nonteaching hospital).
Clinical Case Scenarios
The survey consisted of 7 MeVO narrative cases with illustrative images (4 primary MeVOs and 3 secondary MeVOs) with 3–6 fictional clinical case vignettes each. For the secondary cases of MeVO, presented images were single-image CT (1 section), single-image 3D MRA reconstruction, or angiography snapshots. Clinical data included the fictional patient’s age, stroke severity, relevant medical history, and imaging details (ASPECTS, occlusion location).
One case described an initial carotid-T occlusion, with a secondary embolus in the A3 segment of the anterior cerebral artery after the first thrombectomy attempt. The second case showed an M1 occlusion with a secondary M3/4 occlusion after the first stent-retriever pass. In the third case, CTA showed an M1 occlusion, but only an M2/3 occlusion was present on the first DSA run. The neurologic status of the described patient (improvement, no change, unable to assess due to general anesthesia) varied in each clinical scenario.
For each vignette, the participants were asked if the described patient should be treated endovascularly. Respondents could reply, “Yes, proceed with EVT,” “No, there is no need to treat this occlusion,” or ‘”Wait 10 minutes, repeat DSA, and proceed with EVT if MeVO persists.” If the procedure was initiated with the patient under general anesthesia, there was an additional option to choose, “Wake up the patient and if the symptoms [related to the occlusion territory in question] are present, treat the patient.” The survey flow is shown in the Online Supplemental Data.
Statistical Analysis
Only responses from the cases of secondary MeVO (3 cases with 3 scenarios each) were analyzed in this study. Participants’ baseline characteristics and response data were analyzed using descriptive statistics, and differences between groups were assessed using a χ2 test (binary categoric variables) and a Kruskal-Wallis test (categoric variables with >2 groups).
Univariate logistic regression clustered by respondent identity was used to provide adjusted measures of effect size for baseline characteristics of prespecified scenarios (occlusion site, baseline neurologic deficit) and participant baseline characteristics (participant age and sex, specialty, years of experience in neurointervention, region of practice, personal and center stroke treatment volume per year and hospital setting) on the likelihood of pursuing EVT. Multivariable logistic regression models clustered by respondent identity were additionally performed, including all the aforementioned scenarios and baseline characteristics of the participants. The baseline ASPECTS and patient age were excluded from the multivariable logistic regression model due to the survey-design-related collinearity of these variables.
We reported risk ratios (RRs) derived from binary logistic regression and incidence-rate ratios derived from multivariable Poisson logistic regression. Response options of “No EVT treatment,” “Wait 10 minutes and repeat DSA,” and “Wake up the patient and reassess” were merged into 1 category of “No Immediate EVT,” to determine physician characteristics that were associated with the decision to perform EVT with no delay in procedure continuation. Additionally, we performed an analysis on dichotomized data for “EVT Yes” (merging categories “Proceed with EVT,” “Wait 10 minutes and repeat DSA,” and “Wake up the patient and reassess”) and “EVT No” categories to evaluate participant characteristics associated with a decision in favor of EVT. P values < .05 were considered statistically significant. Changes in decisions between scenarios of varying occlusion sites and neurologic statuses were visualized with Sankey diagrams. Data analysis was performed in STATA 16.1 (StataCorp). Figures were created with Power BI desktop 2016 (Microsoft).
RESULTS
In total, 366 physicians (56 women, 308 men, 2 of undisclosed sex) of different specialties (170 interventional neuroradiologists, 36 interventional neurologists, 18 interventional radiologists, 97 neurologists, 39 neurosurgeons, and 6 other specialists involved in acute stroke care) from 44 countries (49.5% from Europe, 26.2% from the United States and Canada, and 24.3% from the other parts of the world) completed the survey for a total of 3294 responses. Detailed respondent characteristics are listed in the Online Supplemental Data.
EVT in Secondary MeVOs
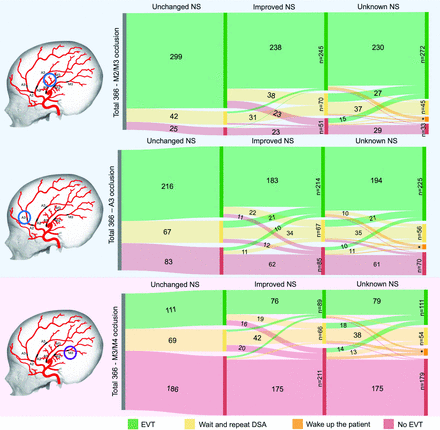
Most physicians (54.1%, 1782/3294 responses) were in favor of continuing with EVT, while 17.9% (589/3294 responses) would wait and repeat the DSA or wake up the patient for neurologic reassessment if the procedure was performed with the patient under general anesthesia. Twenty-eight percent (923/3294 responses) would not have continued with EVT. Participants were more likely to treat patients if the occlusion site was in the anterior M2/3 (74.3%; RR = 2.62; 95% CI, 2.27–3.03) and A3 (59.7%; RR = 2.11; 95% CI, 1.83–2.42) segments, compared with those in the M3/4 segments (28.3%; reference) (Table and Figure). An improvement in neurologic status led to a significant decrease in the likelihood of pursuing EVT compared with statuses of patients whose neurologic deficits remained unchanged (49.9% versus 57.0% responses in favor of EVT, respectively; RR = 0.88; 95% CI, 0.83–0.92). This finding remained statistically significant in cases with M2/3 (RR = 0.82; 95% CI, 0.77–0.87) and M3/4 (RR = 0.80; 95% CI, 0.70–0.92) occlusions, but not for A3 segment occlusions (RR = 0.99; 95% CI, 0.92–1.07) (Table and Figure).
Endovascular decision-making based on patient neurologic status, stratified by site of occlusion
The effect of occlusion site and patient neurologic status (NS) on EVT decision-making for secondary MeVOs. The frequency of physicians’ responses for treatment options in given scenarios with changing neurological status are listed on vertical axis in the diagram. The evolution in physicians’ decision for endovascular treatment based on the changed neurological status is listed in horizontal fashion. Number of responses for category “Wake up the patient”: M2/M3=16; A3=15; M3/4=22. No values are provided for response frequency of <10.
Physician Characteristics and Decision-Making for Immediate EVT
In the univariable analyses, female physicians were less likely to proceed with immediate EVT in comparison with their male counterparts (45.6% versus 55.6%; RR = 0.82; 95% CI, 0.68–0.99). There was a significant difference in treatment decision rates among interventionalists (either interventional neuroradiologists, interventional radiologists, interventional neurologists, or neurosurgeons) compared with noninterventionalists (neurologists and other physicians) (59.7% versus 39.7%, respectively; RR = 1.50; 95% CI, 1.29–1.76).
Physicians working in nonteaching hospitals were more likely to proceed immediately with EVT in contrast to physicians working in teaching hospitals (65.5% versus 53.1%, respectively; RR = 1.23; 95% CI, 1.04–1.46). Those practicing in Europe were less restrictive in their decision to treat patients with MeVO stroke endovascularly (59.3% of responses in favor of EVT; RR = 1.18; 95% CI, 1.01–1.37) in comparison with physicians practicing in the United States and Canada (50.3%) or in other parts of the world (48.0%).
Willingness to immediately proceed with EVT was associated with the physician’s experience in neurointervention (55.4%–59.8% with >5 years’ experience versus 52.1% with 0–5 years’ experience) and their career stage (53.6%–57.8% of board-certified physicians favored immediate EVT versus 37.0% of physicians in training). There was no statistically significant difference in EVT decision-making based on physicians’ ages.
We examined the effect of annual stroke treatment volume, both at the center level and at the personal level, on decision-making in the univariable analysis and found that physicians from centers with >200 mechanical thrombectomies per year (63.2%; RR = 1.42; 95% CI, 1.13–1.79) and physicians performing >50 mechanical thrombectomies annually were more likely to treat patients with MeVO immediately (63.1%; RR = 1.58; 95% CI, 1.01–2.48).
In the multivariable analysis, the physician factors significantly associated with the decision to immediately continue with EVT were specialty (interventionists), region of practice, hospital type, and annual stroke treatment volume of >200 thrombectomies at the center level (Online Supplemental Data).
Physician Characteristics and Decision-Making for EVT (Immediately or after Reassessment)
Most physicians’ baseline characteristics were associated with a preference for proceeding with EVT; rates in favor of EVT ranged from 60% to 77.8%.
In the univariable analyses, interventionalists, those practicing in Europe, and female physicians were more likely to proceed with EVT, either immediately or after reassessment (Online Supplemental Data). In the multivariable analysis, the physician factors significantly associated with the decision to continue with EVT were specialty (interventionists) and region of practice (Europe) (Online Supplemental Data).
Reasons for No EVT
Of 923 no EVT responses (28.0%), most physicians (39.0%, 360 responses) reasoned that the treatment benefit was too small for EVT. The second most frequent reason for no EVT was a too-distal location of the occlusion (31.3%, 289 responses), followed by insufficient evidence that EVT is effective (23.5%, 217 responses). Other reasons such as administration of intra-arterial tPA, good collateral flow, low NIHSS, or high-risk/low-benefit ratio were mentioned by 4.7% of physicians, and insufficient resources to treat MeVOs was chosen by 1.5%.
DISCUSSION
Our findings suggest that a physician’s willingness to treat secondary MeVOs endovascularly is limited and varies per occlusion location. Most participants were in favor of treatment of M2/3 (74.3%) and A3 (59.7%) occlusions, while less than one-third opted to proceed with EVT for the more distally located M3/4 occlusions (28.3%). These data suggest that there is a certain limit to what is perceived as safely achievable and accessible for EVT. Cases that were used to create illustrative scenarios in the survey were successfully achieved and treated endovascularly. Therefore, this finding of the limit based on occlusion location more likely reflects subjective operator experience. Indeed, a too-distal location of the thrombus was the second most frequent reason given by the survey participants for no EVT.
Physicians were less likely to pursue secondary MeVOs if the patient’s clinical status improved in the scenarios in which distal embolization occurred in the initially affected territory (M2/3, M3/4). This scenario is suggestive of a hesitation of physicians to pursue EVT in secondary MeVOs in cases of early neurologic improvement. Furthermore, studies suggest that achieving TICI 2b with fewer passes is associated with better outcome compared with TICI 2c/3 reperfusion achieved with more passes.6 The neurologic status had no impact on the participants’ decisions to treat the presented case of A3 MeVO. This might be due to the initially unaffected anterior cerebral artery territory being supplied by the contralateral ICA. Thus, this MeVO can be considered embolization into a new territory, which may have affected the physician’s willingness to treat.
Physician’s characteristics related to an increased likelihood of immediate EVT in secondary MeVOs were interventional specialty, more experience in neurointervention, and higher annual case volumes. These are likely because interventionalists encounter such cases on a more frequent basis because LVOs migrate distally and become secondary MeVOs.5 In contrast, an average stroke neurologist likely has a limited knowledge of interventional techniques and, therefore, is unable to decide whether a distally located occlusion is accessible for EVT and can be successfully retrieved. Nevertheless, even noninterventionalists and less experienced neurointerventionalists would consider EVT based on either persistent occlusion on repeat DSA or after neurologic reassessment (Online Supplemental Data).
The eloquence of the affected territory plays an important role in EVT decision-making in patients with MeVO stroke. Despite MeVO’s relatively smaller vascular territory in comparison with LVO, the impact of ischemia can be severely disabling,7 for example, the loss of language skills due to a left anterior M2/M3 occlusion. In secondary MeVO strokes, evaluation of a patient’s neurologic status can be challenging because the neurologic deficit might be influenced by the initial LVO stroke.4 In the presented cases of secondary MeVOs in our survey, the most frequent reason for no EVT was that the benefits are too small: The affected areas were not considered responsible for the patients’ neurologic deficits, or the potential risks of the procedure were perceived to be greater than the benefits of the treatment. For example, due to the perceived fragility of the more distal, smaller-caliber vessels, there may be a potential risk of higher complication rates in MeVOs (eg, perforation, intracranial dissection).
Another aspect of a physician’s decision-making is the presence/absence of collateral flow in the affected area. If collateralization behind the occlusion is insufficient, the willingness to pursue distally located occlusions will be higher than in the case of rapid retrograde filling of the vascular bed. As a part of each case presentation, we included an image demonstrating a parenchymal phase on DSA (Online Supplemental Data) to account for this factor; however, a single image cannot replace a complete DSA run, and participants may have thought that they did not have enough information to make a decision.
The accessibility of more distally located occlusions is highly dependent on the available material. In our survey, we did not imply what EVT technique should be used, and it was left to the physician’s own judgment and experience to decide whether the presented occlusion was accessible. The potential challenges of MeVO EVT lie in the progressively changing and generally smaller caliber of the vessels, as well as thinner vessel walls in comparison with the more proximal vessel segments.8 Based on a recent systemic review by Ospel and Goyal,4 the most commonly reported complication in MeVO EVT was symptomatic intracranial hemorrhage. The prevalence of symptomatic intracranial hemorrhage ranges from 0% to 11%, with most studies reporting symptomatic intracranial hemorrhage rates of <8%,4 ie, slightly higher than that reported in the major LVO studies.9 One of the hypothesized mechanisms of periprocedural complications is endothelial injury during stent-retriever expansion and thrombus retrieval.10,11 This could possibly apply even more to fragile, smaller distal vessels, while it has been shown that the radial force of stent retrievers gradually rises as the vessel diameter decreases.10 Smaller and softer devices that can be delivered through smaller microcatheters with an optimized vector of force to avoid pulling the whole vessel along with it are, therefore, warranted.
The optimization of the vector of force can be accomplished using an appropriately sized distal access catheter in conjunction with a stent retriever; such an approach can also decrease the risk of embolization into new territories. With the recent development of longer microcatheters12 that enable delivery of stent retrievers to desired target locations, the option of coaxial delivery of a distal-access catheter over the microcatheters, and recent progress in the development of smaller stent retrievers showing promising results in achieving successful recanalization in MeVOs,13 the perception of the thrombus accessibility is likely to be shifted beyond the M3 or A3 vessel segments, particularly as experience with the novel material further increases.
Our study has limitations. First, the included participants were contacted through personal and professional networks of the study authors, and most (92.1%) were working in teaching hospitals. Therefore, responses may not be representative of the entire stroke community, and ascertainment bias may have occurred while more experienced and skillful interventionalists from high-volume teaching centers were predominantly represented in the survey dataset. However, replies from all over the world were collected, and we particularly assessed differences between regions to support correct local interpretation of the results. Moreover, we believe that the clinical practice in rich countries and teaching institutions serves as exemplary care that is usually followed by the rest of the medical society. Second, survey data do not always reflect real-world clinical practice. Physicians’ choices may be different in practice when facing diverse practical obstacles. Third, participants may have expected that a certain answer to the question was desired from them, probably in favor of EVT, given that the survey is conducted and spread in a research network partly dedicated to improving EVT results. We tried to minimize this effect by making all responses fully anonymous. Fourth, we did not collect information regarding institutional standard stroke imaging protocols and, therefore, did not assess the impact of various imaging protocols on participant decision-making. Because the EVT decision-making was based on the findings of DSA images, the baseline imaging (either CT or MR) played only an illustrative role in the presentation of the secondary MeVO cases. Finally, physicians could have been biased by the vascular anatomy provided for a particular case; our results regarding the occlusion sites are, therefore, not generalizable.
Due to lack of high-level evidence, a decision toward continuing with EVT in secondary MeVOs is mainly made on an individual basis and involves thrombus accessibility, eloquence of the affected territory, a patient’s current condition, and potential risks of the procedure. Therefore, it is clear that physicians vary in their treatment preferences with respect to secondary MeVOs and that experience with such cases is likely to play a role. Despite increasing effort to conduct a randomized controlled trial of EVT in patients with MeVO stroke, it is unlikely that patients with secondary MeVO will be randomized because it is not feasible to randomize patients during an ongoing EVT procedure. However, more evidence on the safety and efficacy of EVT for MeVO stroke is needed, for which observational or survey data could be of great use.
CONCLUSIONS
This study provides valuable information on current practice and EVT decision-making for the challenging-yet-common scenario of secondary MeVO. In the presented scenarios, physicians’ willingness to treat secondary MeVOs endovascularly varied per occlusion location and was influenced by the change in neurologic status.
Acknowledgments
We would like to acknowledge all survey participants for their time and effort invested in filling out the survey, and we thank Moiz Hafeez for his contribution to data entry of the survey scenarios.
Footnotes
P. Cimflova and R. McDonough contributed equally to the manuscript.
Disclosure forms provided by the authors are available with the full text and PDF of this article at www.ajnr.org.
References
- Received June 2, 2021.
- Accepted after revision August 18, 2021.
- © 2021 by American Journal of Neuroradiology