Abstract
BACKGROUND AND PURPOSE: There are only few data and lack of consensus regarding antiplatelet management for carotid stent placement in the setting of endovascular stroke treatment. We aimed to develop a consensus-based algorithm for antiplatelet management in acute ischemic stroke patients undergoing endovascular treatment and simultaneous emergent carotid stent placement.
MATERIALS AND METHODS: We performed a literature search and a modified Delphi approach used Web-based questionnaires that were sent in several iterations to an international multidisciplinary panel of 19 neurointerventionalists from 7 countries. The first round included open-ended questions and formed the basis for subsequent rounds, in which closed-ended questions were used. Participants continuously received feedback on the results from previous rounds. Consensus was defined as agreement of ≥70% for binary questions and agreement of ≥50% for questions with >2 answer options. The results of the Delphi process were then summarized in a draft manuscript that was circulated among the panel members for feedback.
RESULTS: A total of 5 Delphi rounds were performed. Panel members preferred a single intravenous aspirin bolus or, in jurisdictions in which intravenous aspirin is not available, a glycoprotein IIb/IIIa receptor inhibitor as intraprocedural antiplatelet regimen and a combination therapy of oral aspirin and a P2Y12 inhibitor in the postprocedural period. There was no consensus on the role of platelet function testing in the postprocedural period.
CONCLUSIONS: More and better data on antiplatelet management for carotid stent placement in the setting of endovascular treatment are urgently needed. Panel members preferred intravenous aspirin or, alternatively, a glycoprotein IIb/IIIa receptor inhibitor as an intraprocedural antiplatelet agent, followed by a dual oral regimen of aspirin and a P2Y12 inhibitor in the postprocedural period.
ABBREVIATIONS:
- EVT
- endovascular treatment
- GPIIb/IIIa
- glycoprotein IIb/IIIa
Currently, it is not clear whether and when carotid stent placement should be performed in patients with acute ischemic stroke with extracranial carotid stenosis, occlusion, or unstable plaques undergoing endovascular treatment (EVT), but there is no doubt that carotid stent placement is necessary in some cases.1 Numerous studies and review articles discuss the benefits and disadvantages of carotid stent placement in the setting of EVT,1⇓-3 and the need for dual-antiplatelet therapy is often cited as an argument to forego emergent stent placement. Some authors even argue that emergent carotid endarterectomy might be a better alternative because it does not require dual antiplatelet therapy.4 There are only few data on antiplatelet management for carotid stenosis in the setting of conservative management,5 and even less is known about periprocedural antiplatelet management for carotid stent placement during EVT:6 There are no comprehensive studies that have compared the impact of different antiplatelet regimens on clinical outcomes and hemorrhagic events in patients who undergo EVT and simultaneous carotid stent placement.
Answering these questions is, however, of utmost importance because ischemic brain tissue is at higher risk of hemorrhage, and this risk may further increase with suboptimal antiplatelet management, especially if intravenous alteplase is administered concurrently.7,8 The prevailing uncertainty regarding timing, dosage, and agents in antiplatelet therapy for emergent carotid stent placement might: 1) influence the decision about whether to place a carotid stent in a disadvantageous manner (ie, no carotid stent is placed in patients who could benefit from stent placement), and 2) compromise patient safety through increased thromboembolic or hemorrhagic complications in case a carotid stent is placed. In addition, the lack of standardization makes it difficult to perform unbiased retrospective studies. We used a modified Delphi approach to identify current challenges and unsolved questions in antiplatelet management for emergent carotid stent placement in the setting of EVT and attempted to propose a consensus-based algorithm for standardized antiplatelet management strategies until evidence-based guidelines become available. Of note, the question of whether and when carotid stent placement should be considered in a patient undergoing EVT was not the subject of this study.
MATERIALS AND METHODS
Literature Search
In preparation for the Delphi process, a MEDLINE literature search using the search terms “antiplatelet,” “emergent,” “acute,” “carotid stent placement,” “thrombectomy,” “endovascular,” and “stroke” was performed for the period from January 2010 to May 2020. Bibliographies of relevant publications were screened to identify additional studies. Together with the results from round 1 (open-ended questions), the identified articles provided the basis for the following, closed-ended survey questions.
Panel Members
A panel of 19 neurointerventionalists (interventional neuroradiologists, vascular neurosurgeons, and vascular neurologists) from Europe, North America, Asia, and Africa with high clinical and academic expertise in endovascular stroke treatment was formed on the basis of personal and institutional academic and clinical collaborations, and care was taken to represent a broad spectrum of specialties and countries. Panel members were selected only if they had long-standing clinical experience and scientific interest in endovascular stroke treatment. A list of the panel members can be found in the Online Appendix. In addition, a pharmacology expert (L.T.) with intraprocedural antiplatelet management experience in neurointervention was consulted and provided feedback.
Delphi Methodology
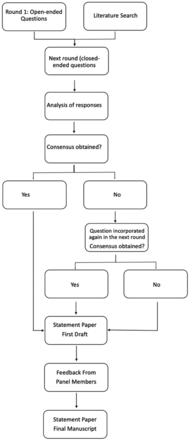
The Delphi method was originally developed to predict the impact of technology on warfare during the cold war.9 Delphi is a systematic and iterative forecasting method with interactive feedback loops that relies on a panel of individuals with high expertise in the area of interest. It is generally used when reliable data for a particular question of interest are not available. The panel undergoes a series of questionnaires with controlled-opinion feedback, whereby the ultimate goal is to reach a group consensus. The Delphi method has been successfully used in medicine to develop temporary treatment guidelines and to standardize patient care in areas with a relative paucity of data.10 In this study, the Delphi technique was applied to identify challenges in antiplatelet management for carotid stent placement in the setting of EVT and to develop a proposed algorithm for such cases. Figure 1 outlines the principal steps of the Delphi approach as it was performed in this study.11
Delphi methodology as it was used in this article.
Data Collection and Analysis
An anonymous online response system (Qualtrics.com) was used for all survey rounds. While the anonymization prevented us from performing stratified analysis, eg, by expert specialty or region of practice, we considered it necessary to blind the panel members as well as the data analysts to the results to avoid biases due to peer pressure. The 19 panel members responded independently from each other to subsequent iterations of questionnaires. The initial round contained exclusively open-ended questions. Answers from this round were thematically clustered and analyzed in an affinity diagram. The literature search results and the affinity diagram formed the basis for the following rounds, which consisted of closed-ended questions. An anonymized result summary from the previous round was fed back to the group during the next round, and group responses were assessed for consensus. One question was thereby asked a maximum of 2 times.
RESULTS
Data Collection and Endorsement
Response data were collected from March 31 to May 6, 2020. All 19 panel members completed a total of 5 survey rounds, in which they were asked to answer questions regarding antiplatelet management for carotid stent placement in the setting of EVT according to their personal experience and views, ie, their answers represented personal beliefs rather than established policies at their local institutions. The results were summarized in an initial document, which was circulated among the panel members for further discussion before finalizing the manuscript. The statement was endorsed by the World Federation of Interventional and Therapeutic Neuroradiology, the Japanese Society for Neuroendovascular Therapy, and the Chinese Neurosurgical Society.
Literature Search
The literature search revealed wide practice variations in antiplatelet management for carotid stent placement in the setting of EVT. Numerous studies reported the safety and efficacy with regard to clinical outcomes of the procedure itself, but the specific antiplatelet regimen used was reported in only very few studies. In those that did report it, antiplatelet protocols ranged from rather aggressive protocols with glycoprotein IIb/IIIa (GPIIb/IIIa) inhibitors and additional aspirin to no periprocedural antiplatelet therapy at all12 (see Online Table for key publications). Most studies were retrospective in nature, and among those with uniform antiplatelet regimens, most were small single-center series, while in larger multicenter studies, antiplatelet regimens were not reported or varied among patients.13
Delphi Consensus Results
The panel achieved a consensus that antiplatelet management for carotid stent placement in the setting of EVT should follow a standard approach, irrespective of the baseline ASPECTS/ischemic “core” on perfusion imaging and the final reperfusion result (expanded TICI score), and that it should be independent of whether intravenous thrombolytics (alteplase, tenecteplase) are administered. They also believed that heparin, other than small doses in the infusion, should not be administered during the procedure.
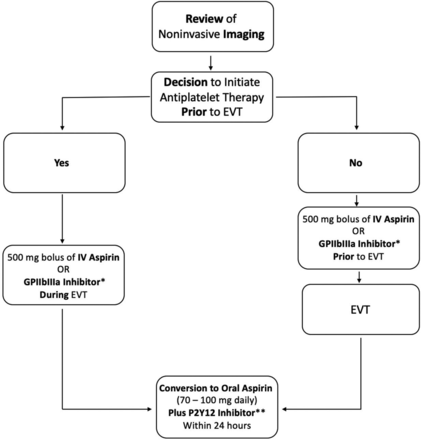
Panel members did not achieve consensus on whether antiplatelet therapy should be initiated on the basis of noninvasive imaging findings (head and neck CTA) before the procedure if there is a perceived high likelihood of a carotid stent becoming necessary (eg, high-grade carotid stenosis). Thus, in the following, the results are reported separately for 2 scenarios: 1) patients in whom the likelihood of a carotid stent being needed seems high; therefore, the operator decides to initiate antiplatelet therapy before the procedure; and 2) patients in whom antiplatelet therapy has not been initiated before the start of the procedure but is initiated during the procedure.
Scenario 1: Initiation of Antiplatelet Therapy before the Procedure
In case the likelihood of a carotid stent being placed is so high that the operator decides to initiate antiplatelet therapy before the EVT procedure, panel members agreed that aspirin should be used as a first-line agent (500 mg bolus) and is sufficient; a second periprocedural antiplatelet agent was not deemed necessary. In case intravenous aspirin is not available, most panel members favored GPIIb/IIIa inhibitors (see Table 1 for dose recommendations) or rectal aspirin as an alternative.
Consensus recommendations for the dosage of GPIIb/IIIa receptor inhibitorsa
Scenario 2: Initiation of Antiplatelet Therapy during the Procedure
If an operator decides to initiate antiplatelet therapy during the procedure because of carotid stent placement, the panel also preferred intravenous aspirin as a first-line agent (500 mg bolus) without any additional antiplatelet agents. In case intravenous aspirin is not available, GPIIb/IIIa inhibitors were deemed the most suitable alternative (see Table 1 for dose recommendations).
Postprocedural Antiplatelet Management
Panel members agreed that the intraprocedural intravenous regimen can be converted to an oral regimen within 24 hours after the EVT procedure. Oral aspirin (70–100 mg, depending on the available dosages in individual jurisdictions) was, the preferred first antiplatelet agent in the postprocedural period. P2Y12 inhibitors were the preferred second agent (see Table 2 for dose recommendations). It was believed that particularly in case of known clopidogrel resistance, it may be beneficial to choose another P2Y12 inhibitor. Regarding the usefulness and clinical impact of antiplatelet testing in the postprocedural period, no consensus was achieved. Figure 2 provides a short summary of the panel consensus.
Summary of the results of the Delphi consensus panel. The asterisk means see Table 1 for dosages. Two asterisks mean see Table 2 for dosages.
Consensus recommendations for dosing of oral P2Y12 inhibitors in the postprocedural perioda
DISCUSSION
Panel members in this Delphi study reached to a consensus on a single-antiplatelet regimen with intravenous aspirin or alternatively an intravenous GPIIb/IIIa inhibitor as a first-line approach for carotid stent placement in the setting of EVT, irrespective of whether antiplatelet therapy is initiated prior to or during the procedure. The preferred oral antiplatelet regimen in the postprocedural period was a dual regimen with aspirin and a P2Y12 inhibitor. No consensus was achieved on the role of platelet function testing.
Our literature search revealed a lack of high-level evidence for antiplatelet management for emergent carotid stent placement because most studies were small single-center series. Many publications did not report the antiplatelet regimen that was used at all, while in others, it was reported but not standardized. This leads to substantial uncertainty on the side of neurointerventionalists who have to decide whether to place a carotid stent during a thrombectomy procedure and might result in undertreatment, ie, a carotid stent is not placed though it is needed, or suboptimal antiplatelet regimens that increase the risk of either hemorrhagic or thrombembolic complications. Intravenous aspirin is a long-standing antiplatelet agent that has been used by neurointerventionalists in Europe for many years. It was the preferred agent for intraprocedural antiplatelet management in this study. However, intravenous aspirin is not available in North America and some other countries. In such cases, the panel believed that a GPIIb/IIIa inhibitor would constitute the best alternative. Panel members stated that they would use these antiplatelet regimens, irrespective of whether intravenous thrombolytics are administered, though the Antiplatelet therapy in combination with rtPA Thrombolysis in Ischemic Stroke (ARTIS) trial7 and some other studies8 have shown that the risk of bleeding in patients with acute ischemic stroke who receive intravenous alteplase is increased when antiplatelet therapy is initiated immediately. In the postprocedural period, the panel preferred an oral combination therapy with aspirin and a P2Y12 inhibitor, which is very similar to the standard antiplatelet regimen after elective carotid stent placement.
Of note, this consensus is largely based on the panel members’ experience rather than evidence, and there are large practice variations in antiplatelet management for emergent carotid stent placement.12,14 Thus, the antiplatelet dosages on which the panel members agreed are also not in any way evidence-based and are largely extrapolated from existing literature on elective carotid stent placement and personal experience. The lack of consensus regarding platelet function testing is most likely due to poor standardization of commercially available test kits and contradictory evidence regarding their utility in the setting of neurovascular procedures.15,16 Panel members clearly emphasized the need for more and better, ideally prospective and multicentric, studies on the impact of antiplatelet regimens on hemorrhagic/thromboembolic events and outcomes in carotid stent placement during EVT, similar to the currently ongoing Multicenter Randomized CLinical trial of Endovascular treatment for Acute ischemic stroke in the Netherlands investigating the effect of periprocedural MEDication (MR CLEAN MED) trial (https://www.mrclean-med.nl), which evaluated the effect of heparin and/or antiplatelet agents in the setting of EVT (but without simultaneous carotid stent placement). They also pointed out the need for better standardization of and evidence for platelet function testing and the development of antithrombogenic surface coatings that could in the long run obviate the need for systemic antiplatelet therapy altogether.
Limitations
The results of this Delphi consensus study are intended to identify challenges and unsolved questions in antiplatelet management for emergent carotid stent placement and to provide a possible antiplatelet management approach until sufficient data become available that allow evidence-based recommendations. This article does not intend, in any way, to replace such guidelines; on the contrary, its goal is to encourage investigators to initiate these urgently needed studies. It should also not be misinterpreted as advocating carotid stent placement in the setting of EVT; this question will hopefully be answered soon by randomized trials such as the Thrombectomy In TANdem occlusion (TITAN) trial (NCT03978988) (https://clinicaltrials.gov/ct2/show/NCT03978988), and it is not our intention to promote one antiplatelet agent over another.
Only a few countries being represented in the panel could have led to nongeneralizability of the results, eg, with regard to different geographic regions because region-specific access limitations to drugs could not be fully taken into account. This feature was, in part, related to the number of experts, which was rather small. We decided to stick to 15 experts, a number that is commonly used in the Delphi method because the chances of achieving group consensus decrease rapidly with larger group sizes. Last, antiplatelet agents and application forms are constantly refined and new ones are developed, and antithrombogenic device surface coatings could soon find their way into clinical practice17,18 so that the panel consensus might look different if this study were to be repeated in the future.
CONCLUSIONS
More and better data on antiplatelet management for carotid stent placement in the setting of EVT are urgently needed. Expert panel members in this study preferred intravenous aspirin or, alternatively, a GPIIb/IIIa inhibitor as an intraprocedural antiplatelet agent, followed by a dual oral regimen of aspirin and a P2Y12 inhibitor in the postprocedural period.
Footnotes
Disclosures: Mayank Goyal—UNRELATED: Consultancy: Stryker, Medtronic, Mentice, MicroVention; Grants/Grants Pending: Cerenovus*; Patents (Planned, Pending or Issued): GE Healthcare, Comments: licensing agreement for systems of stroke diagnosis. Shinichi Yoshimura—RELATED: Consulting Fee or Honorarium: Bayer, Sanofi, Otsuka Pharmaceutical, Stryker, Medtronic, Johnson & Johnson, Termo, Medico’s Hirata, Biomedical Solutions, Comments: lecture fee; UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Boehringer-Ingelheim, Daiichi Sankyo, Bristol-Meyers Squibb, Comments: lecture fee. Jens Fiehler—UNRELATED: Consultancy: Acandis, Cerenovus, Stryker, Medtronic, Penumbra; Grants/Grants Pending: Acandis, Cerenovus, Stryker, Medtronic, Route 92.* Mahesh Jayaraman—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Medtronic, Comments: speaking at ISC 2018 on Stroke Systems of Care. Franziska Dorn—UNRELATED: Consultancy: Balt, phenox, Cerus; Payment for Lectures Including Service on Speakers Bureaus: speakers honorary for Cerenovus, Acandis. Allan Taylor—RELATED: Other: limited private practice; UNRELATED: Employment: Groote Schuur Hospital; Expert Testimony: defense and prosecuting attorneys. Raul Nogueira—Other Relationships: RGN reports, consulting fees for advisory roles with Anaconda, Biogen, Cerenovus, Genentech, Imperative Care, Medtronic, phenox, Prolong Pharmaceuticals, Stryker Neurovascular; Stock/Stock Options: advisory roles with Astrocyte, Brainomix, Cerebrotech, Ceretrieve, Corindus Vascular Robotics, Vesalio, Viz-ai, and Perfuze. Justin F. Fraser—UNRELATED: Board Membership: Cerelux LLC, Comments: co-owner of company; Consultancy: Medtronic, Penumbra, Stream Biomedical, Comments: consultant fees; Grants/Grants Pending: American Heart Association and University of Kentucky, Comments: grant support*; Stock/Stock Options: Fawkes Biotechnology, Comments: equity interest ownership. Rene Chapot—UNRELATED: Consultancy: MicroVention, Stryker*; Payment for Development of Educational Presentations: Balt, MicroVention, Siemens.* Charles Majoie—UNRELATED: Grants/Grants Pending: CVON/Dutch Heart Foundation, European Commission, Dutch Health Evaluation Program, TWIN Foundation, Stryker*; Stock/Stock Options: Nico.lab (modest), Comments: a company that focuses on the use of artificial intelligence for medical image analysis. David Kallmes—UNRELATED: Grants/Grants Pending: MicroVention, NeuroSigma, Medtronic, Comments: funding for preclinical research*; Patents (Planned, Pending or Issued): Mayo Clinic, Comments: patent for protection device*; Stock/Stock Options: Marblehead Medical LLC, Superior Medical Experts LLC. Patrick Brouwer—UNRELATED: Consultancy: Cerenovus. Nobuyuki Sakai—UNRELATED: Grants/Grants Pending: Daiichi Sankyo, NeuroVasc, Terumo*; Payment for Lectures Including Service on Speakers Bureaus: Asahi-Intec, Biomedical Solutions. Adam Arthur—UNRELATED: Consultancy: Balt, Johnson & Johnson, Medtronic, MicroVention, Penumbra, Scientia, Siemens, Stryker*; Grants/Grants Pending: Balt, Medtronic, MicroVention, Penumbra, Siemens*; Stock/Stock Options: Azimuth, Bendit, Cerebrotech, EndoStream, Magneto, Marblehead Medical LLC, Neurogami, Serenity, Synchron, TRIAD MEDICAL, Vascular Simulations.* *Money paid to the institution.
References
- Received June 1, 2020.
- Accepted after revision July 17, 2020.
- © 2020 by American Journal of Neuroradiology