Abstract
SUMMARY: When preparing for the coronavirus disease 2019 pandemic and its effects on the CNS, radiologists should be familiar with neuroimaging appearances in past zoonotic infectious disease outbreaks. Organisms that have crossed the species barrier from animals to humans include viruses such as Hendra, Nipah, Severe Acute Respiratory Syndrome, and influenza, as well as bacteria and others. Brain CT and MR imaging findings have included cortical abnormalities, microinfarction in the white matter, large-vessel occlusion, and features of meningitis. In particular, the high sensitivity of diffusion-weighted MR imaging in detecting intracranial abnormalities has been helpful in outbreaks. Although the coronaviruses causing the previous Severe Acute Respiratory Syndrome outbreak and the current coronavirus disease 19 pandemic are related, it is important to be aware of their similarities as well as potential differences. This review describes the neuroimaging appearances of selected zoonotic outbreaks so that neuroradiologists can better understand the current pandemic and potential future outbreaks.
ABBREVIATIONS:
- COVID-19
- coronavirus disease 2019
- HeV
- Hendra virus
- MERS-CoV
- Middle East respiratory syndrome coronavirus
- NiV
- Nipah virus
- SARS-CoV-2
- Severe Acute Respiratory Syndrome coronavirus 2
Currently, the coronavirus disease 2019 (COVID-19) pandemic is sweeping across the world, caused by the emerging novel zoonotic virus Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2).1 First detected in Wuhan, China, in late 2019, COVID-19 (the clinical syndrome caused by the virus) has resulted in high mortality and morbidity, overwhelming the health services as well as causing severe disruption to the economy in many parts of the world.1,2 In the past 20 years, there have been increasing epidemic outbreaks of zoonotic diseases, including coronaviruses and various other pathogens.3,4 Zoonoses often present with neurologic symptoms and imaging changes on CT and MR imaging; hence, it is important for neuroradiologists to be familiar with the imaging findings in past outbreaks in addition to obtaining up-to-date information on the current one.5,6 This review is based on a literature review and the authors’ ongoing research and introduces the zoonotic infections and associated neuroimaging appearances in past outbreaks that have affected the CNS, to improve our understanding of the current pandemic and future zoonotic disease outbreaks.
Emerging Zoonotic Diseases and the CNS
A zoonotic disease is defined as an infectious agent leaping from an animal reservoir to humans, often caused by mutations that permit accessibility through receptor or immune barriers of the novel host.7 In the broadest sense, zoonoses encompass both direct spread from a vertebrate animal to humans, as well as vector-borne diseases that use intermediate arthropod vectors (typically mosquitos, ticks, sand flies, and so on), resulting in indirect spread to humans. Additionally, zoonotic pathogens range from viruses, bacteria, parasites, and fungi to prions.8 The global burden of animal-to-human disease transmission is increasing, and up to 76% of emerging infectious disease events in recent years have been zoonotic in nature.9 Historically, the 3 pandemics of greatest significance—the Plague (or “Black Death”), the 1918 Spanish flu, and HIV/AIDS—were all caused by zoonoses or vector-borne diseases, leading to hundreds of millions of deaths worldwide.10
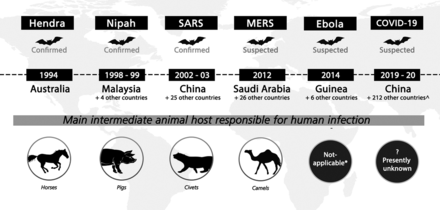
In recent decades, bats have emerged as mammalian hosts serving as reservoirs for pathogens of high mortality. Although historically known to transmit rabies virus alongside the order Carnivora, bats have now been shown to carry assorted viruses from different families, including zoonotic viruses such as Coronaviridae, Filoviridae, and Paramyxoviridae, among others.11 Bats are also a well-recognized reservoir for Lyssaviruses, including the rabies virus and Australian bat lyssavirus, which classically result in encephalitic or paralytic rabies.12 Critically, bat-borne viruses cause regular and cyclical outbreaks via zoonotic transmission and spillover into secondary mammalian hosts. Increasing recognition of bats as animal reservoirs of zoonotic pathogens began with the Hendra virus (HeV) outbreak in 1994 and has only since accelerated up to the current COVID-19 pandemic.13,14 Other important examples of novel zoonotic virus emergence linked to bats include the 1998 Nipah virus (NiV) disease in pigs in Malaysia, Severe Acute Respiratory Syndrome coronavirus 1 (SARS-CoV) in China in 2002, Middle East respiratory syndrome coronavirus (MERS-CoV) in Saudi Arabia starting in 2012, and devastating outbreaks of filoviruses from 1967 through 2018 (Fig 1).15,16
Summary of major emerging zoonotic outbreaks related to bats, 1994–2019. Confirmed bat-borne viruses include Hendra, Nipah, and SARS-CoV-1 viruses; bats are also suspected to be viral reservoirs for MERS, Ebola, and SARS-CoV-2 (cause of the current COVID-19 pandemic) viruses. Years when outbreaks occurred and confirmed or suspected intermediate hosts involved in virus spillover are also shown. * indicates that although the 2014 Ebola outbreak was believed to have started with direct bat-to-human transmission, nonhuman primates have been implicated in previous Ebola outbreaks. ∧Data accurate as of May 11, 2020. Reprinted with permission of Duke-NUS Medical School.
Table 1 shows examples of zoonotic diseases that have been associated with CNS manifestations. Zoonotic diseases with other animal hosts include the 2009 outbreak of zoonotic “swine flu” influenza H1N1 strain and sporadic human cases of avian influenza subtypes H5N1 and H9N2.17 Within rodent reservoirs in Europe, the Americas, and Asia, hantaviruses have been found to spill over into humans and may also present with pulmonary and neurologic symptoms.18 A large number of CNS infections are vector-borne diseases, notably arthropod-borne Alphaviruses and Flaviviruses. Key examples include the mosquito-borne Japanese encephalitis virus and West Nile virus, dengue, Chikungunya, Zika (responsible for an outbreak in 2015–2016 that caused fetal MR imaging changes),19 and tick-borne viruses. A review of vector-borne CNS diseases is outside the scope of this article, which focuses on zoonotic outbreaks directly from animals to humans. Notably, most recent zoonotic outbreaks reaching regional or global significance appear to be viral in nature.
Key zoonotic diseases with CNS manifestations
Neuroimaging in Recent Zoonotic Infectious Outbreaks Affecting the CNS
Zoonotic Bat-Borne Henipaviruses: Hendra and Nipah Viruses.
A separate genus of Henipavirus within the Paramyxoviridae family was created after the discovery of 2 new viruses causing zoonotic outbreaks, which were named after the HeV and NiV. HeV (initially named equine morbillivirus) was isolated during 2 outbreaks in 1994 at a stable in Hendra, a suburb of Brisbane, Australia, causing the deaths of 16 horses, a trainer, and another animal handler.20 Ongoing HeV outbreaks in Australia have been reported, with a total of 94 equine disease cases, 7 human cases, and 4 deaths as of 2015, typically in veterinarians or animal handlers, with a fatality rate of 80% in horses and 57% in humans.21,22 The virus presents with an acute febrile respiratory disease and neurologic symptoms (eg, confusion, ataxia, and seizures) in humans. Brain MR imaging showed widespread cortical lesions with sparing of the subcortical white matter on T2-weighted images, similar in appearance to subacute sclerosing panencephalitis caused by measles, the prototypic paramyxovirus.21,23
NiV, named after the village where the virus was first isolated, caused a larger pig-borne outbreak of fatal encephalitis and pneumonia in 1998. A total of 265 cases of acute NiV encephalitis with 105 deaths were recorded in Malaysia.24 The virus crossed the border to neighboring Singapore, which imported live pigs for slaughter, and spread to 11 slaughterhouse workers, of whom 1 died.25,26 Patients typically presented with fever, vomiting, headache, and dizziness, which developed into severe encephalitis with altered consciousness, brain stem dysfunction, seizures, and myoclonic jerks.26,27 Neurologic involvement was diverse, including aseptic meningitis, diffuse encephalitis, and focal brain stem and cerebellar involvement. Some early cases were initially ascribed to Japanese encephalitis virus, which had previously caused pig-associated outbreaks in Malaysia, but there were many clinical, epidemiologic, and radiologic red flags that helped to differentiate between the diseases.
As transmission was from bats to pigs and subsequently pigs to humans, pig farms were shut and pigs culled in Malaysia. All importation of live pigs and pork products to Singapore from Malaysia was banned, and slaughterhouses closed, ending the outbreak in 1999. Starting in 2001, multiple NiV outbreaks were reported in West Bengal, India, and multiple locations in Bangladesh, with local epidemics spreading as far southward and westward as Kerala.24 These outbreaks were caused by direct transmission from bats to humans (via consumption of fresh date palm sap or fruit contaminated by bat secretions or from contact with infected animals) and human-to-human spread of infection (including health care workers).28 NiV outbreaks mainly caused encephalitis, but respiratory symptoms have been reported in from 29% to two-thirds of cases in different outbreaks.29 More recent reports of outbreaks in Mindanao, the Philippines (fatal human encephalitis, influenza-like disease, and meningitis) were spread by diseased horses.30
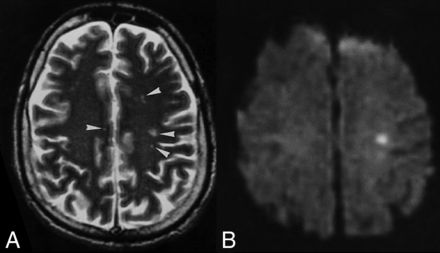
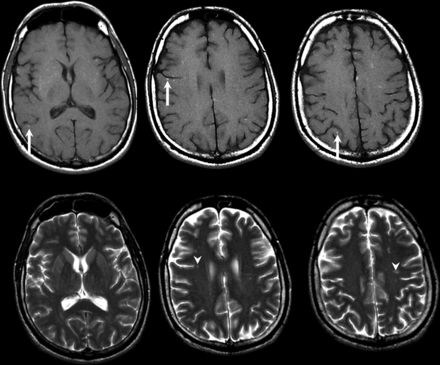
MR imaging among Singaporean patients infected with NiV showed multifocal, bilateral tiny (<1 cm in maximum diameter) abnormalities, more within the subcortical and deep white matter (Fig 2) than the cortex, brain stem, or corpus callosum. Most were prominently detected by DWI compared with T2-weighted images, and some lesions enhanced after contrast media injection.31,32 In a follow-up MR imaging study among patients in Singapore, multiple small T1-weighted hyperintensities in the cerebral cortex, similar in appearance to laminar cortical necrosis, were noted 1 month after the outbreak, but there were no clinical or radiologic relapse or subacute sclerosing panencephalitis features. These tiny T1-weighted abnormalities also disappeared on later MR imaging studies at 6 months and later (Fig 3). Among a cohort of asymptomatic-but-seropositive Singapore slaughterhouse workers who were exposed to pigs, delayed MR imaging revealed discrete tiny lesions in the brain (but without DWI abnormalities at the late time of acquisition) similar to those detected in symptomatic patients with encephalitis.33 In the patients in Singapore who were followed-up, 1 patient developed myelopathic symptoms from a cervical spinal cord lesion; a proportion developed psychiatric features, including depression, personality changes, and deficits in attention and memory.
Patient with Nipah virus infection: initial infection. A, Multiple punctate white matter lesions (arrowheads) are visible on T2-weighted FSE MR image. B, The largest lesion is more prominent on corresponding DWI. Images reprinted from Lim et al.31
Patient with Nipah virus infection 1 month after infection. Selected axial T1-weighted images (upper row) show multifocal punctate high signal intensity on the cortical surfaces (arrows) as well as in the white matter. These did not enhance after contrast injection and disappeared on 6-month and subsequent follow-up MR imaging (not shown). Selected T2-weighted images (lower row) show noncorresponding multiple tiny focal increased signal intensity (arrowheads) in the white matter. These also became smaller or disappeared on follow-up MR imaging (not shown).
In contrast, follow-up of patients in Malaysia showed a different neuroimaging pattern of extensive patchy and confluent involvement of the cortex, temporal lobe, and pons on T2-weighted images.34 In a series of 160 Malaysian survivors of Nipah encephalitis, 12 patients had relapses, and 3 had late-onset encephalitis.35⇓-37 Malaysian patients may have been more severely affected than those in Singapore, perhaps because of the nature of their exposure to the virus.29 The Singapore MR imaging pattern of tiny DWI abnormalities followed by transient T1 hyperintensities was distinctly different from the characteristic features of herpesvirus and Japanese encephalitis virus and was more consistent with virus-associated microangiopathy and ischemic microinfarction. Postmortem studies performed on Malaysian patients showed both direct neuronal invasion and disseminated microinfarction from vasculitis-induced thrombosis. Small and medium-sized blood vessels in other organs also had similar vasculitic lesions, resulting in endothelial multinucleated syncytia and fibrinoid necrosis.24,38 In addition, postmortem examination of 2 fatal cases of HeV infections in Australia showed similar findings, which suggested that the pathologic mechanisms might be similar in HeV and NiV and supporting ischemic microinfarction as a partial explanation for MR imaging findings.39,40
Zoonotic Bat-Borne Coronavirus: SARS-CoV and MERS-CoV
SARS-CoV and MERS-CoV are 2 newly discovered β-coronaviruses and have much more aggressive behavior than the 4 endemic α-coronaviruses and discovered β-coronaviruses, which are known causes of the common cold.41 Severe Acute Respiratory Syndrome (SARS) emerged in 2002 in Guangdong province, China, most probably spread via civet cats as intermediate hosts. Human-to-human transmission and global travel rapidly caused the outbreak to spread to a total of 33 nations. SARS was predominantly an atypical pneumonia with lower respiratory tract infection with limited transmissibility as a rule, yet punctuated by a few superspreading events, and affected 8096 people with 774 deaths.42 The SARS outbreak ended in 2003, and although a small number of cases have occurred as a result of laboratory accidents or through animal-to-human transmission, there have been no large-scale SARS outbreaks since.43
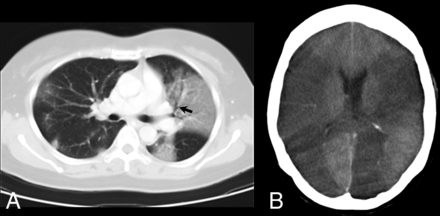
CNS manifestations were noted in several seriously ill patients with multiple complications.44 Of the 238 patients with SARS in Singapore, 5 had CNS complications, 4 patients were critically ill, and 3 died.45 CT performed in 4 patients showed cerebral infarction involving the large-artery territories (Fig 4), including the middle cerebral artery in all 4 (bilateral in 1) and posterior cerebral artery in 2; hemorrhagic conversion was noted in 1 patient.45 An increased incidence of pulmonary embolism and deep venous thrombosis was also noted, especially among critically ill patients treated with intravenous immunoglobulin, raising the possibility of a prothrombotic effect. Hence, the role of hypercoagulable state, iatrogenic measures, systemic hypotension, and cardiac dysfunction in seriously ill patients with SARS has been proposed to explain these findings; increased vigilance against stroke and other thrombotic complications in future outbreaks has been recommended.45
Patient with SARS infection. A, Axial 10-mm-section portable chest CT scan shows multifocal and confluent ground-glass opacities bilaterally with air bronchogram (arrow) in the left upper lobe. B, Axial CT scan of the head shows extensive low-attenuation cerebral infarction involving bilateral middle cerebral and left posterior cerebral artery territories.
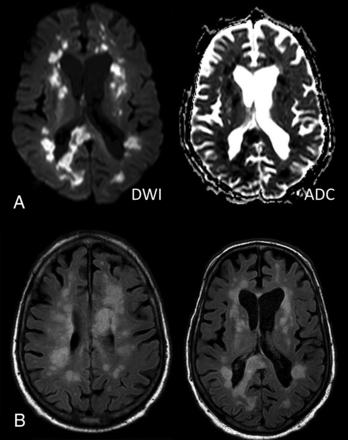
MERS coronavirus, with camels as intermediate hosts, emerged in 2012 in the Middle East and spread to 26 other nations.15 A total of 2121 patients were affected in Saudi Arabia, the epicenter of the initial outbreak, and 2519 total cases and 866 deaths have occurred globally since then, with a high case fatality ratio around 35%.46 MERS-CoV presents similar to SARS-CoV in the form of severe respiratory distress, which may progress to shock, acute kidney injury, and coagulopathy. MERS outbreaks in most countries have subsided after infection-control measures but are still simmering with sporadic cases, necessitating maintained hospital vigilance and travel-history screening.46 In 1 patient interpreted as having acute disseminated encephalomyelitis, brain MR imaging on day 28 of illness revealed widespread, bilateral, nonenhancing hyperintense lesions on DWI and T2-weighted imaging within the frontal, temporal, and parietal subcortical white matter and corpus callosum (Fig 5); other findings included 1 patient with bilateral anterior cerebral artery infarction and another with intracranial hemorrhage.47,48 Four of 23 patients affected by the 2015 outbreak in Korea had neurologic symptoms during or after MERS-CoV treatment. These included Bickerstaff encephalitis overlapping with Guillain-Barré syndrome and critical illness neuropathy and myopathy; abnormal MR imaging findings were not reported.49
Patient with MERS-CoV infection (on day 28). A, DWI and ADC mapping show diffusion restriction of the multiple white matter lesions. B, Axial FLAIR images show multiple hyperintense lesions in the subcortical areas and deep white matter of the frontal, temporal, and parietal lobes bilaterally as well as in the corpus callosum. Images reprinted from Arabi et al.47
Zoonotic Viruses from Putative Bat Hosts and Other Animal Reservoirs
Both Ebola virus and Marburg virus are members of the Filoviridae family, several of which cause severe hemorrhagic fevers.50,51 Extensive surveillance studies have identified bats as a primary reservoir and nonhuman primates as intermediate hosts, with human consumption of bushmeat implicated in localized outbreaks.16 Since their discovery, at least 28 Ebola virus outbreaks and 13 Marburg virus outbreaks have occurred in sub-Saharan Africa, often complicated by armed conflict and hostility to medical support teams; hence, neuroimaging is poorly described.51 Patients with Ebola virus typically have bleeding complications, and CNS involvement includes a case report of meningoencephalitis in a seriously ill patient with multiple organ failure. DWI showed multiple punctate lesions in the corpus callosum, cerebral white matter, and spinal cord, reported as consistent with microvascular occlusion and ischemia. There were no hemorrhagic lesions, and results of Ebola virus polymerase chain reaction testing on CSF were negative.52
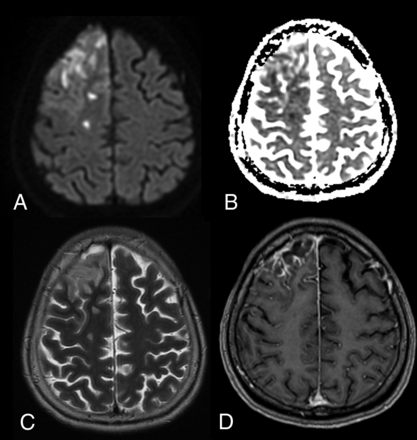
Apart from bat-borne zoonotic viruses, novel influenza A viruses, including those that normally circulate in pigs (H1N1, H1N2, H3N2), and the highly virulent avian H5N1 and H7N9 viruses, are always threatening to trigger the next pandemic.53 However, the recent 2009 influenza A H1N1 pandemic did not result in widespread CNS abnormalities, though a few reports have described brain CT or MR imaging findings. Notably, several reports described bilateral thalamic lesions, some with DWI high signal and focal hemorrhage, consistent with acute necrotizing encephalopathy (Fig 6), as well as patients with meningeal enhancement.54,55 New-onset seizures and encephalopathy were found to commonly occur in patients with underlying neurologic disorders, with swelling of bilateral basal ganglia and thalami and cerebral edema and tonsillar herniation noted on MR imaging.56 Overall, viruses remain the most likely class of organisms resulting in zoonotic outbreaks affecting the CNS.
Patient with influenza A H1N1 infection. A, Axial T2-weighted image shows symmetric increased signal intensity in the thalami and supratentorial frontal white matter. B, T2-weighted gradient-echo image reveals decreased signal intensity in the central portion of the thalami, indicating hemorrhagic necrosis. C, Axial DWI reveals restricted diffusion, with a concentric pattern, symmetrically involving the thalami. Images reprinted from Ormitti et al.54
Zoonotic Outbreak from Contaminated Raw Fish: Group B Streptococcus ST283
Group B streptococcus bacteria are common gastrointestinal and genitourinary commensal organisms in humans, and they can cause bacteremia and meningitis in neonates and pregnant women. Invasive group B streptococcus infection is rare in healthy adults, but among patients with chronic underlying comorbidities,57 it can cause urinary tract and soft-tissue infection, osteomyelitis, infective endocarditis, and pneumonia, but rarely meningitis.58 In 2015, there was an outbreak of 238 cases of group B streptococcus infections in a foodborne outbreak associated with consumption of Chinese-style raw freshwater fish59; 29 patients had meningoencephalitis, fever, meningism, headache, encephalopathy, focal neurologic deficits, and/or seizures.
The invasive serotype III sequence type 283 (ST283) Streptococcus agalactiae was identified as the causative organism, with the same strain identified among farmed freshwater fish used for food preparation.60⇓-62 The outbreak stopped after affected fish imports into Singapore were banned and stricter rules were established governing raw fish dishes.60 However, there have been other, smaller outbreaks of human meningitis in Hong Kong and Southeast Asia involving the ST283 strain, suggesting a potential public health threat of this foodborne zoonosis in Asia.63 Other bacteria have caused smaller zoonotic outbreaks in the past, including meningitis from infected unpasteurized dairy products, but the ST283 outbreak demonstrates the need for vigilance and a broader perspective of CNS involvement in zoonotic disease.64
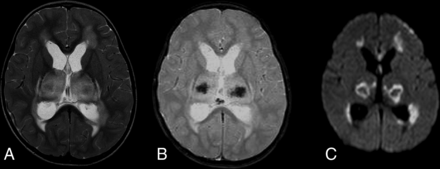
DWI showed single or multiple tiny hyperintensities in the subarachnoid space or ventricles, consistent with small amounts of pus (Fig 7).62,65 Eight of 14 patients also had DWI lesions in the basal ganglia, corona radiata, thalamus, midbrain, cerebral peduncle and corpus callosum, posterior limb of the internal capsule, frontal and parietal cortex, and white matter; 5 had cerebellar involvement.65 Hyperintense pus detected by DWI in the subarachnoid space is nonspecific; many bacteria, tuberculosis, or fungi can be responsible. Meningitis may also cause additional MR imaging features such as meningeal enhancement, abscess formation, or hydrocephalus.66 However, DWI demonstrated high sensitivity, and tiny subtle abnormalities were either less conspicuous or not visualized on T1- or T2-weighted images, suggesting cerebral infarction as a possible mechanism for some of the unusual brain parenchymal involvement.65
Patient with group B Streptococcus ST283 meningitis. A, Axial DWI shows multifocal high signal intensity in the cortex, white matter, and subarachnoid space of the right frontal lobe. B, ADC map shows corresponding low ADC. C, T2-weighted images show increased signal. D, After contrast injection, T1-weighted images show leptomeningeal enhancement, typical of meningitis.
Observations and Lessons Learned from Past Zoonotic Outbreaks
Zoonotic and nonzoonotic infections can affect the CNS by different mechanisms, and neuroradiologists may observe 3 broad patterns of changes on CNS imaging in serious infections. First, there may be direct injury to nervous tissue, entering via hematogenous or neuronal invasion and causing damage to the brain and meninges (eg, herpes virus and Streptococcus).41 The causative bacterium or virus is usually isolated from the CSF, as has been described in HeV and NiV outbreaks; however, in many case reports for SARS-CoV/SARS-CoV-2, MERS-CoV, and the Ebola virus, the causative viruses were not successfully isolated.45,47 Second, an immune-mediated response may be responsible for some MR imaging findings, including an early excessive innate immune response such as “cytokine storm” (eg, acute necrotizing encephalopathy similar to cases described in H1N1)67 and a late immune response, typically taking place some days or weeks after an acute infection (eg, acute disseminated encephalomyelitis and Guillain-Barré syndrome in the peripheral nervous system).68 Finally, CT and MR imaging manifestations may be the result of cerebrovascular complications, pre-existing comorbidities, or iatrogenic effects; these are often seen in critically ill patients in the intensive care unit. Thus, the imaging findings may not be easily categorized or definitively explained because individual patients may sustain CNS damage from multiple mechanisms.6
Neuroradiologists must be aware of CNS imaging features of past zoonotic infections to be prepared for current and future pandemics. In this review, we have highlighted CNS imaging in several past zoonotic infectious outbreaks, summarized in Table 2. The patterns of brain abnormalities include widespread cortical or multifocal white matter lesions, large arterial territory infarction, hemorrhage, and meningeal enhancement. Some may be relevant to the current COVID-19 pandemic, and some may eventually turn out not to be relevant or even misleading. Knowledge of typical MR imaging features of bacterial meningitis enabled authors to identify tiny amounts of pus in the subarachnoid space and ventricles in the ST283 group B Streptococcus outbreak with high sensitivity, and knowledge of Japanese encephalitis virus features enabled researchers to eliminate it during the NiV outbreak. Being open to the possibility of vasculitis-induced and coagulopathic effects of infection enabled the observations in NiV and SARS. DWI is extremely sensitive to small lesions, though it is challenging to distinguish between ischemic and infective brain damage; however, in an outbreak of infectious disease, the challenges of transportation and acquiring MR imaging in severely ill and intubated patients may mean that CT may be more appropriately used in these situations.45,69 Finally, newer MR imaging pulse sequences such as susceptibility-weighted imaging and perfusion MR imaging may contribute to the original research literature on COVID-19, which is still being written.
Key brain neuroimaging findings in zoonotic outbreaks
CONCLUSIONS
In military history, generals and commanders are cautioned against “fighting the last war” by using outdated equipment, tactics, and concepts and failing to realize that their enemies and situations have changed. Martial language is often used to describe the current pandemic as a war, with troops, frontlines, and victories. Although Mark Twain famously said that history may not repeat itself (in exactly the same manner), it certainly has many common features. Neuroradiologists should recognize the MR imaging and CT imaging features of past zoonotic outbreaks but be prepared to recognize new imaging manifestations as data emerge. As health care professionals worldwide labor to suppress the COVID-19 pandemic, neuroradiologists should not be fighting the last war; we should instead unite and learn together in this unprecedented shared struggle.5,70
Acknowledgments
The authors thank Ms Qianhui Cheng, Mr Shawn Teo Yi En, Dr T. Umapathi, and Dr Ng Yuen Li for their kind contributions.
Footnotes
Disclosures: Lin-Fa Wang—RELATED: Grant: National Research Foundation, Comments: Grants for research from 2013 to 2019.* *Money paid to the institution.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received May 19, 2020.
- Accepted after revision June 15, 2020.
- © 2020 by American Journal of Neuroradiology