Abstract
SUMMARY: The sinonasal tract is an environment diverse with neoplasia. Given the continued discovery of entities generally specific to the sinonasal tract, the fourth edition of the World Health Organization Classification of Head and Neck Tumors was released in 2017. It describes 3 new, well-defined entities and several less-defined, emerging entities. The new entities are seromucinous hamartomas, nuclear protein in testis carcinomas, and biphenotypic sinonasal sarcomas. Emerging entities include human papillomavirus–related sinonasal carcinomas, SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1–deficient sinonasal carcinomas, renal cell-like adenocarcinomas, and chondromesenchymal hamartomas. The literature thus far largely focuses on the pathology of these entities. Our goal in this report was to familiarize radiologists with these new diagnoses and to provide available information regarding their imaging appearances.
ABBREVIATIONS:
- HPV
- human papillomavirus
- NUT
- nuclear protein in testis
- NMC
- nuclear protein in testis midline carcinomas
- SCC
- squamous cell carcinoma
- SH
- seromucinous hamartoma
- SWI/SNF
- SWItch/Sucrose Non-Fermentable
- SMARCB1
- SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1
- SNUC
- sinonasal undifferentiated carcinoma
- REAH
- respiratory epithelial adenomatoid hamartoma
- WHO
- World Health Organization
The complex anatomy and histology, numerous potential carcinogenic exposures, and vast cancer genomics cause the aerodigestive tract to be affected by a diverse range of tumors and tumor-like entities. To stay current, the classification of head and neck tumors is frequently redefined. The fourth edition of the World Health Organization Classification of Head and Neck Tumors, released in 2017, includes 3 new, well-defined entities and several less-defined, emerging entities within the sinonasal tract. The emerging entities are provisional diagnoses or are only described in the context of differential diagnoses.1,2
The new sinonasal entities are seromucinous hamartomas, nuclear protein in testis (NUT) carcinomas, and biphenotypic sinonasal sarcomas. Emerging entities include SWI/SNF-related matrix-associated actin-dependent regulator of chromatin subfamily B member 1 (SMARCB1)–deficient sinonasal carcinomas, renal cell-like adenocarcinomas, and chondromesenchymal hamartomas. Human papillomavirus (HPV)-related sinonasal carcinoma with adenoid cystic features is described as an emerging entity within the World Health Organization (WHO) update. A subsequently published, expanded series suggests that this entity may be a distinct one, termed “HPV-related multiphenotypic sinonasal carcinoma.”3
To date, the literature regarding these updates largely concerns the histopathologic features.2,4,5 A radiologist may see these rare entities reported as pathologic diagnoses, and some familiarity with the nomenclature, pathology, and imaging features is important. This article aims to briefly review the histopathologic classifications of sinonasal tumors as well as provide imaging examples and reported imaging characteristics of these new and emerging entities. While there are no true pathognomonic imaging features and biopsy remains the criterion standard, there are findings that may be useful to characterize these lesions.
Review of Histologic Types of Sinonasal Tumors
Accurate interpretation of sinonasal tumor imaging by neuroradiologists necessitates a fundamental understanding of the histologic subtypes. Basic classification groups include squamous cell carcinomas (SCCs), adenocarcinomas, sarcomatous/mesenchymal tumors, neuroectodermal tumors, salivary neoplasms, papillomas, respiratory epithelial lesions, hematolymphoid tumors, and tumor-like entities (On-line Table).
Squamous cell carcinomas are defined as keratinizing and nonkeratinizing subtypes.6 Nonkeratinizing SCCs account for about 15%–20% of sinonasal SCCs.4 Under the umbrella of nonkeratinizing SCC is the emerging subgroup of HPV-related sinonasal carcinoma.1,7
Sinonasal adenocarcinomas are classified as either intestinal or nonintestinal subtypes.8 Nonintestinal types are either high- or low-grade.9,10 Emerging within the subgroup of low-grade nonintestinal is one that resembles conventional clear cell adenocarcinoma, thus termed “renal cell-like adenocarcinoma.”1,2
Other histologic carcinoma groups include neuroendocrine tumor, lymphoepithelial carcinoma, sinonasal undifferentiated carcinoma (SNUC) including the emerging subtype of SMARCB1-deficient sinonasal carcinoma, and the newly defined NUT carcinoma.1,2,11 Biphenotypic sinonasal sarcoma is a newly defined sinonasal sarcomatous/mesenchymal tumor. New tumor-like entities include seromucinous hamartomas and chondromesenchymal hamartomas.1
New Entities
Seromucinous Hamartoma
Clinical and Histopathologic Review.
Sinonasal epithelial hamartomas are benign lesions that contain components of normal ciliated respiratory epithelium. Subtypes include respiratory epithelial adenomatoid hamartoma (REAH) and the newly defined seromucinous hamartoma (SH).12⇓⇓–15 SH has increased seromucinous glandular components compared with REAH, akin to adenosis.16
Both lesions affect adults with a male predilection, 7:1 for REAH and 5:2 for SH. Presenting symptoms for both include nasal congestion, anosmia, and rhinorrhea.15,17 REAHs have been described in isolation and in the setting of concomitant sinonasal disease from inflammatory polyposis to malignancy, an association that suggests a potential reactive etiology of these lesions.18,19 Recurrence of REAHs and SHs after resection is rare.16
Imaging Features.
Just as there is histologic overlap of REAH and SH, their imaging features are similar. REAHs classically arise in the olfactory clefts. Other described sites for both lesions include the nasal septum, middle turbinate, and uncinate process. The lesions may be bilateral.16,17,20
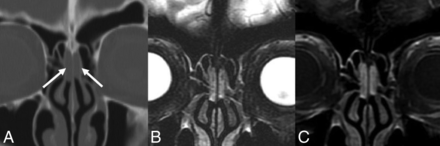
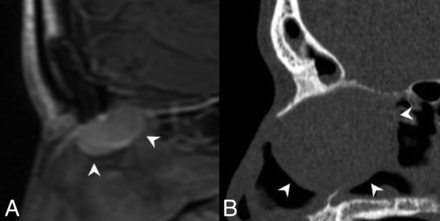
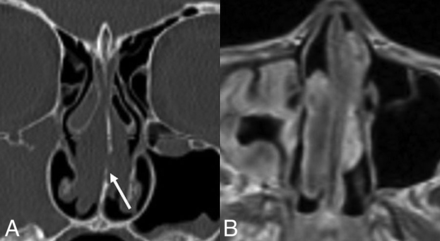
REAHs are well-defined, near muscle density, and homogeneous on CT. The olfactory cleft is often expanded and smoothly remodeled without erosive changes.18 REAHs are heterogeneous on T2WI and isointense on T1WI relative to the cortex and enhance uniformly (Fig 1).21 To our knowledge, no substantial dedicated literature regarding the appearance of SHs on imaging exists to date. Published CT images in the pathology literature show an appearance similar to that of REAH.20 MR images of a proved SH published here show similar signal on T2WI and enhancement patterns (Fig 2). On the sagittal images, both often have a characteristic half-moon appearance when situated in the olfactory cleft, here described as a “crescent sign” (Fig 3).
REAH. A, Coronal noncontrast CT shows mildly expansile soft tissue in the bilateral olfactory clefts without adjacent bony erosion or destruction (white arrows). Coronal T2 fat-saturated (B) and coronal T1 postcontrast, fat-saturated (C) MR images in the same patient show the lesions to be heterogeneous and hyperintense to the cortex on T2WI and enhancing.
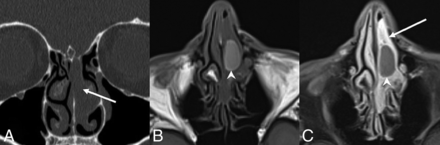
SH. A, Coronal noncontrast CT shows expansile soft tissue in the left olfactory recess extending inferiorly into the superior nasal cavity (white arrows) without erosive or destructive bony changes, similar to an REAH. Axial T1 precontrast (B) and axial T1 postcontrast, fat-saturated (C) images demonstrate predominantly homogeneous enhancement. The relatively hyperintense, nonenhancing central component on T1WI suggests proteinaceous content (white arrowheads), possibly reflecting glandular secretions. Areas of relative central hyperintense signal on precontrast T1WI are not described in other published examples.
Crescent sign. A, Sagittal T1 postcontrast MR image of an REAH. B, Sagittal noncontrast CT image of a seromucinous hamartoma demonstrates a characteristic half-moon morphology (white arrowheads), which we have termed a “crescent sign” when the lesions arise in the olfactory recess.
Diagnostic Tips and Differentials.
Differentiating REAH from SH is not possible by imaging alone. The presence of frank bony erosion or skull base defects would favor encephalocele, carcinoma, or esthesioneuroblastoma (other soft-tissue lesions typical of the olfactory cleft) over REAH, SH, or benign inflammatory polyp.22 The “crescent sign” morphology on sagittal images, in our experience, is suggestive of REAH and SH within the olfactory cleft.
NUT Carcinoma
Clinical and Histopathologic Review.
Nuclear protein in testis midline carcinomas (NMCs) were initially described in the mediastinum and more recently in the sinonasal cavity. Although there are <100 cases reported, the clear genomics and histology allow the sinonasal NMCs to be included as a new entity in the latest WHO release.23⇓–25 NMCs show undifferentiated round blue cells growing in the submucosa abruptly juxtaposed to mature keratinizing squamous cells.26,27 The diagnosis requires identification of the NUTM1 gene rearrangement, often with BRD4.28
Sinonasal nuclear protein in testis midline carcinoma is highly aggressive and infiltrative, with a poor prognosis and a median progression-free survival of 6.6 months and an overall median survival of 9.7 months.29 Presenting symptoms include nasal obstruction, epistaxis, and orbital pain with a wide age range at presentation and a slight female predominance.25,30
Imaging Features.
While no substantial literature regarding imaging of sinonasal NMCs exists, case reports and images here published show aggressive features similar to those within the chest, where there is often airway and vascular invasion.31 In the sinonasal cavity, rapid growth and infiltration may result in orbital or cranial involvement. Nearly half have regional or distant metastases at presentation.25,27,32
On CT, the mass is near muscle density. Bony hyperostosis is described.31,32 In the case published here, there are internal mineralizations but no dystrophic calcifications as seen in thoracic NMCs.31 On MR imaging, the tumors are hypointense on T1WI and heterogeneous on T2WI relative to the cortex and enhancing (Fig 4).33 Published cases are FDG-avid.34
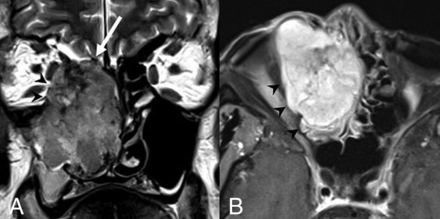
NUT carcinoma. A, Axial noncontrast CT image shows a locally destructive mass centered within the left ethmoid sinus extending into the left orbit and laterally displacing the medial rectus muscle. There is faint mineralization within the mass (white arrowheads). Axial T2 fat-saturated (B) and coronal T1 postcontrast, fat-saturated (C) MR images from the same patient show the mass to be heterogeneous but predominantly hypointense to the cortex on T2WI and avidly enhancing. The mass extends into the anterior cranial fossa with dural involvement (white arrows). Images are courtesy of Dr Nafi Aygun, Johns Hopkins Hospital.
Diagnostic Tips and Differentials.
The locally aggressive imaging characteristics of sinonasal NMCs are almost entirely nonspecific, with a multitude of potential differentials, including other carcinomas, lymphomas, and sarcomas. PET/CT may play an important role in staging, given the propensity for distant metastases at presentation.33
Biphenotypic Sinonasal Sarcoma
Clinical and Histopathologic Review.
Biphenotypic sinonasal sarcomas are histologically similar to cellular schwannomas or malignant peripheral nerve sheath tumors, with infiltrating spindle cells and entrapped invaginations of normal sinonasal epithelium.34⇓–36 Diagnosis requires the rearrangement of the PAX3 gene, often with MAML3.35,37,38
With about 50 reported cases, there is a suggested female predominance (2–3:1), often occurring in the sixth decade.2,35 Described throughout the sinonasal tract, the most common locations of tumors include the superior nasal cavity and ethmoid sinuses, and they present with facial pressure and nasal obstruction.40 The tumors are slow growing with no published cases with metastases to date. Local recurrence, however, is reported in about half of the cases.35,39
Imaging Features.
The masses are well-marginated but locally aggressive and can erode through the skull base or into the orbit. Associated hyperostotic bone formation is described on CT.35,40 The masses avidly enhance and are isointense-to-hypointense to the cortex on T2WI (Fig 5).
Biphenotypic sinonasal sarcoma. Coronal T2 (A) and axial T1 postcontrast, fat-saturated (B) MR images show a well-marginated but locally aggressive right nasoethmoid mass involving the right maxillary sinus. The mass extends through the right lamina papyracea to involve the right orbit (black arrowheads) and through the right lateral lamella and fovea ethmoidalis into the right anterior cranial fossa (white arrow). Heterogeneous but predominantly isointense signal relative to the cortex on T2WI and avid contrast enhancement are noted.
Diagnostic Tips and Differentials.
Exceedingly rare with nonspecific imaging findings, biphenotypic sinonasal sarcomas have imaging findings similar to those of many other primary sinonasal carcinomas and sarcomas. Hyperostotic bone formation is described in several of the published cases but is again nonspecific.35
Emerging Entities: Provisional & Differential Diagnoses
HPV-Related Sinonasal Carcinoma
Clinical and Histopathologic Review.
The sinonasal cavity is a common location within the aerodigestive tract for HPV-related carcinomas. Twenty-to-thirty percent of sinonasal carcinomas have associated high-risk HPV subtypes.2,7,41 Sinonasal HPV-related carcinoma remains an emerging entity because the histopathology is less clear and no definitive prognostic benefit to HPV association is known as in the oropharynx. Preliminary evidence is at least suggestive of improved disease-free and overall survival.7,41,42
There are several known HPV-related sinonasal carcinoma variants (squamous, papillary, small-cell, adenosquamous, basaloid). One particular variant is only described in the sinonasal tract: HPV-related multiphenotypic sinonasal carcinoma.3,7 At the time of the WHO update publication, this variant was called HPV-related carcinoma with adenoid cystic-like features, given the associated microcystic pseudoductal spaces. It was a provisional entity with only 9 documented cases.7 A recent expanded series of 49 cases describes a more complex histopathology with multiple subphenotypes and proposes a change from a provisional diagnosis to a distinct entity renamed “HPV-related multiphenotypic sinonasal carcinoma.”3
Arising in adults, the multiphenotypic variant commonly presents with nasal congestion and epistaxis. Around 90% of the documented cases originate in the nasal cavity. Distant metastases are described in 2 patients, 1 to the lung and 1 to a finger. Approximately 25% have local recurrence.43
Imaging Features.
Little is published on the imaging findings of HPV-related sinonasal carcinomas as a group. Pathology-proved cases here published are similar to those of other sinonasal SCCs destroying, remodeling, and/or invading bone. They have variable signal relative to the cortex on T2WI and enhance on MR imaging (Figs 6 and 7). The multiphenotypic variant case shown here was FDG-avid.
HPV-related sinonasal carcinoma, a nonmultiphenotypic variant. Coronal T2 (A) and axial T1 postcontrast, fat-saturated (B) MR images show a right nasoethmoid mass with extension to the right maxillary sinus and orbit (black arrowheads) as well as the left nasal cavity. There is anterior extension into the right nasal vestibule (white arrows). The mass demonstrates heterogeneous hyperintensity relative to the cortex on T2WI and avidly enhances. Images are courtesy of Dr Nafi Aygun, Johns Hopkins Hospital.
HPV-related multiphenotypic sinonasal carcinoma. Axial noncontrast CT (A) and coronal T1 postcontrast, fat-saturated (B) MR images show an aggressive mass centered in the inferior nasal cavity. The mass is isodense to muscle with an aggressive periosteal reaction along the nasal septum (black arrowhead). The mass is enhancing and locally destructive, invading the marrow of the hard palate (white arrows).
Perineural tumor spread is uncommon in the multiphenotypic variant, unlike true adenoid cystic carcinoma.2,3 No cystic nodal metastases have yet been described.43
Diagnostic Tips and Differentials.
HPV-related sinonasal carcinoma variants, including the multiphenotypic type, share many imaging characteristics with one another and with other primary sinonasal carcinomas (SCC, adenocarcinoma, and so forth). Cystic nodal metastases common in HPV-related carcinomas elsewhere in the aerodigestive tract and perineural spread common to true adenoid cystic carcinomas have not yet been described.2,3,43
SMARCB1-Deficient Sinonasal Carcinoma
Clinical and Histopathologic Review.
SMARCB1-deficient sinonasal carcinomas are composed of mitotically active epithelioid nests with basaloid features and necrosis similar to SNUC and nonkeratinizing SCC.44⇓–46 Given this histologic overlap, SMARCB1-deficient sinonasal carcinomas may represent a distinct entity or a histologic pattern seen in various other tumors.11,47 Diagnosis is by immunohistochemical analysis demonstrating complete loss of SMARCB1 expression, a tumor-suppressor gene implicated in several tumors with rhabdoid features.44,45,47,48 SMARCB1-deficient sinonasal carcinoma is an emerging entity currently classified as a SNUC subtype.
SMARCB1-deficient sinonasal carcinomas are more common in women, with a wide age range from young adult to elderly.11,48 Local recurrence is common. Frequently with local or distant metastases at diagnosis, approximately 40% of cases are lethal.2
Imaging Features.
In an imaging analysis of 17 cases, 8 were centered within the nasoethmoid region.49 The tumors are highly aggressive and infiltrative, many demonstrating local skull/brain invasion. Eight cases had dural involvement, with extension through the dura in 3 cases.49
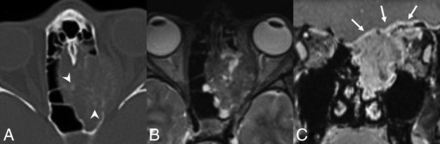
On CT, the tumors are isodense to muscle, with calcifications in half of the reported cases. Both expansile and erosive patterns of bony involvement along the skull base are described with a “hair on end” pattern of periosteal reaction in several cases.49 On MR imaging, most lesions are isointense on T1WI and variable on T2WI relative to the cortex, predominantly avidly enhancing with moderately restricted diffusion (Fig 8).49 All cases were FDG-avid.49
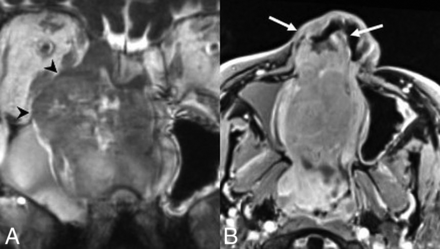
SMARCB1-deficient sinonasal carcinoma. A, Coronal noncontrast CT shows the “hair on end” pattern of calcification/periosteal reaction (white arrows) described in several published cases. Coronal postcontrast CT (B) and coronal T1 postcontrast, fat-saturated (C) MR images in a second patient demonstrate an enhancing nasoethmoid mass eroding the floor of the anterior cranial fossa (black arrows). There is also erosion of the right lamina papyracea with extraconal orbital extension. A secondary mucocele extends through the left orbital roof (white arrowhead).
Diagnostic Tips and Differentials.
Within the broad differential for locally aggressive sinonasal tumors, including SNUC and other carcinomas, SMARCB1-deficient sinonasal carcinoma diagnosis is dependent on tissue sampling. Calcifications are a commonly found imaging feature.
Renal Cell-Like Adenocarcinoma
Clinical and Histopathologic Review.
Renal cell-like adenocarcinoma is a variant of low-grade nonintestinal sinonasal adenocarcinoma with uniform cuboidal/columnar cells with glycogen-rich cytoplasm, akin to clear-cell renal cell carcinoma.4,50 With approximately 16 reported cases, it is an emerging entity in the latest WHO edition. The key differential diagnosis is renal cell carcinoma metastasis. In fact, the most common carcinoma to metastasize to the sinonasal tract is renal cell carcinoma.51 The distinguishing features are immunohistochemical, with renal cell-like adenocarcinoma negative for PAX8, a renal cell carcinoma marker, and vimentin.4,51,52
Renal cell-like adenocarcinoma commonly presents with epistaxis, with ages ranging from early adulthood to elderly and a female predominance (11:5).51,53 The tumor demonstrates indolent growth, and no local recurrence has yet been described.51
Imaging Features.
Published cases show expansile, locally destructive masses with intracranial extension and dural involvement described. On MR imaging, the tumors are heterogeneous to the cortex on T2WI and enhance avidly, similar to renal cell carcinoma.54⇓–56 A key diagnostic criterion is the lack of a concomitant suspicious renal mass (Fig 9).
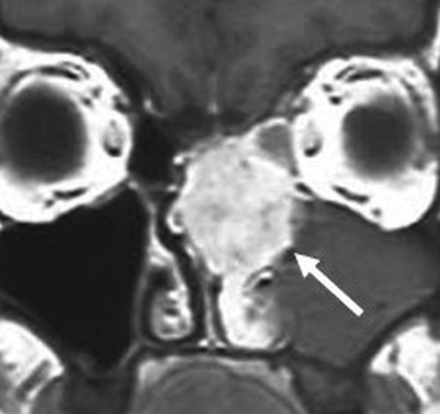
Renal cell-like adenocarcinoma. Coronal T1 postcontrast MR image shows an avidly enhancing, locally destructive, left nasoethmoid mass. The pronounced enhancement is akin to that of a renal cell carcinoma metastasis (white arrow). Images are courtesy of Dr Margie Brandwein, Mount Sinai Hospital.
Diagnostic Tips and Differentials.
There are no pathognomonic imaging findings, and the differential is broad, including other aggressive primary and secondary lesions. The absence of a concomitant renal mass is key to diagnosing renal cell-like adenocarcinoma when tissue sampling is suggestive.
Chondromesenchymal Hamartoma
Clinical and Histopathologic Review.
Chondromesenchymal hamartoma is an emerging tumor-like entity in children. There is a link to germline or somatic DICER1 gene mutations, which are also associated with pleuropulmonary blastoma tumor predisposition disorder.57 Histology demonstrates nodules of hyaline cartilage within the stromal components of spindle cells.58,59
The tumor is benign but locally aggressive, involving the paranasal sinuses, nasal cavities, and orbits. It is more common in male children with a mean age of 9 years. Nine of 42 cases in 1 series had local recurrence. One documented case had malignant transformation.59
Imaging Features.
The masses are smoothly marginated and expansile, commonly with cystic components and calcifications on CT. Erosion of the skull base is often demonstrated. Half of the cases have internal calcifications. On MR imaging, solid components strongly enhance (Fig 10).60,61
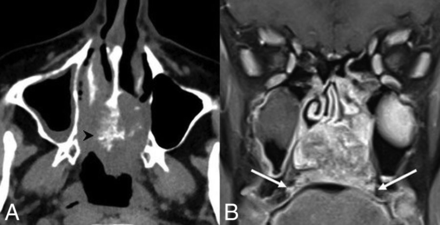
Chrondromesenchymal hamartoma. A, Coronal noncontrast CT in a pediatric patient shows polypoid soft tissue in the bilateral nasal cavities with mild erosive changes focally along the left margin of the nasal septum (white arrow). B, Axial T1 postcontrast, fat-saturated MR image demonstrates heterogeneous enhancement. No cystic or calcified components, frequently described in these lesions, are seen in this case. Images are courtesy of Dr Nafi Aygun, Johns Hopkins Hospital.
Diagnostic Tips and Differentials.
Overlapping imaging findings with other expansile benign-appearing pathology make chondromesenchymal hamartoma a histologic diagnosis but it should be considered in a mixed cystic and calcified sinonasal mass in a child.
Conclusions
The fourth edition of the World Health Organization Classification of Head and Neck Tumors defines several new well-defined and other emerging sinonasal tumor and tumor-like entities. While some of these lesions, including NMCs, biphenotypic sinonasal sarcomas, HPV-related multiphenotypic sinonasal carcinomas, and seromucinous hamartomas, are considered distinct, many others lack clearly defined histopathology and genomics and are thus considered emerging or provisional diagnoses.1⇓–3
In the olfactory cleft, REAH and SH often take on the herein described “crescent sign” morphology on sagittal images. Otherwise, these new and emerging entities have similar, locally destructive appearances on imaging. Certain entities have more typical features, such as calcifications with SMARCB1-deficient sinonasal carcinomas and cystic and calcified components in chondromesenchymal hamartomas, but no pathognomonic findings exist. These entities are rare, many exceedingly so, and should generally not be standard in a typical sinonasal mass differential. However, familiarity with these diagnoses is warranted. As more imaging examples of these entities are analyzed, further clarification of any specific imaging characteristics may be useful in refining these differentials.
Acknowledgments
We would like to thank Dr Nafi Aygun, Johns Hopkins Hospital; Dr Caroline Robson, Boston Children's Hospital; Dr Christine Glastonbury, University of California, San Francisco; and Dr Margaret Brandwein-Weber, Mount Sinai Hospital, for providing excellent sample images.
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- Received October 26, 2018.
- Accepted after revision December 20, 2018.
- © 2019 by American Journal of Neuroradiology