Abstract
BACKGROUND AND PURPOSE: In patients with hemorrhagic contusions, hematoma volumes are overestimated on follow-up standard 120-kV images obtained after contrast-enhanced whole-body CT. We aimed to retrospectively determine hemorrhagic progression of contusion rates on 120-kV and 190-keV images derived from dual-energy CT and the magnitude of hematoma volume overestimation.
MATERIALS AND METHODS: We retrospectively analyzed admission and follow-up CT studies in 40 patients with hemorrhagic contusions. After annotating the contusions, we measured volumes from admission and follow-up 120-kV and 190-keV images using semiautomated 3D segmentation. Bland-Altman analysis was used for hematoma volume comparison.
RESULTS: On 120-kV images, hemorrhagic progression of contusions was detected in 24 of the 40 patients, while only 17 patients had hemorrhagic progression of contusions on 190-keV images (P = .008). Hematoma volumes were systematically overestimated on follow-up 120-kV images (9.68 versus 8 mm3; mean difference, 1.68 mm3; standard error, 0.37; P < .001) compared with 190-keV images. There was no significant difference in volumes between admission 120-kV and 190-keV images. Mean and median percentages of overestimation were 29% (95% CI, 18–39) and 22% (quartile 3 − quartile 1 = 36.8), respectively.
CONCLUSIONS: The 120-kV images, which are comparable with single-energy CT images, significantly overestimated the hematoma volumes, hence the rate of hemorrhagic progression of contusions, after contrast-enhanced whole-body CT. Hence, follow-up of hemorrhagic contusions should be performed on dual-energy CT, and 190-keV images should be used for the assessment of hematoma volumes.
ABBREVIATIONS:
- DECT
- dual-energy CT
- HPC
- hemorrhagic progression of contusion
- SECT
- single-energy CT
- WBCT
- whole-body CT
Approximately 6 million patients with head and neck trauma are seen annually in the emergency departments of North America.1 One of the most severe types of traumatic brain injury is cerebral hemorrhagic contusion.2 Hemorrhagic contusions are usually complicated by secondary injury, resulting in hemorrhagic progression of contusion (HPC), which is designated as enlargement of the existing hemorrhagic contusions or appearance of new lesions.2 Several authors have reported rates of HPC in 38%–59% of cases.3⇓⇓⇓⇓–8 HPC is a progressive injury that results in irreversible loss of brain tissue with significant increase in morbidity and mortality.3 Noncontrast head CT is the diagnostic method used to assess patients with hemorrhagic contusions and HPC.3
There is increasing availability of dual-energy CT (DECT) technology in major academic and level 1 trauma centers. We have included a high-monochromatic (190-keV) image set in our routine head CT protocol at the University of Maryland Shock Trauma Center and Adult Emergency Department due to its potential to positively affect the display of cortical contusions and subdural hematomas by decreasing the beam-hardening artifacts from cranial bones.9⇓–11 Mixed 120-kV images derived from dual-energy data mirror typical single-energy CT (SECT) images used in clinical practice. We have frequently observed higher hemorrhagic contusion volumes on follow-up 120-kV compared with 190-keV images (Fig 1A, -B). This discrepancy was seen in patients after admission contrast-enhanced whole-body CT (WBCT) imaging, which has become a widely used technique for the work-up of the patient with blunt polytrauma.12
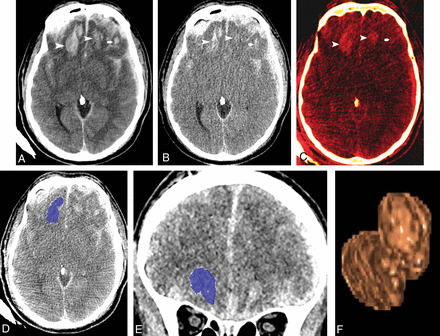
A 55-year-old man with traumatic brain injury sustained after an assault. A, Follow-up axial 120-kV image shows hemorrhagic contusions in both frontal lobes (arrowheads and arrow). B, Virtual high-monochromatic image (190-keV) shows significantly smaller hemorrhagic contusions compared with the 120-kV images due to negligible attenuation contribution from leaked iodinated contrast (arrowheads and arrow). C, Iodine overlay image shows contrast staining of the hemorrhagic contusions (arrowheads and arrow). Axial (D) and coronal (E) CT images demonstrate the ROIs drawn by 3D segmentation on the thin-client server. F, Segmented volume-rendered image of the hemorrhagic contusion.
Studies have shown an increase in capillary endothelial permeability in contusions and surrounding parenchyma.2 We hypothesized that the discrepancy in measured volumes on follow-up noncontrast head CT is caused by retained iodinated contrast that leaks into the parenchyma through the permeable endothelium in the epicenter and penumbra of the contusions after admission contrast-enhanced WBCT (Fig 1C). The high attenuation of the leaked iodine resembles that of hematoma on 120-kV images, resulting in volume overestimation.13⇓–15 The linear attenuation of iodine shows a dramatic decrease along the spectrum of monochromatic energy, therefore greatly reducing the attenuation contribution of iodine at 190 keV, while at the same time, maintaining the attenuation of hematoma relatively constant.16,17 Hence, at clinically relevant tissue iodine concentrations, the attenuation contribution of iodine tends to be negligible at 190 keV and demonstrates only the attenuation resulting from hematoma, thus allowing true measurement of hematoma volume.13,16,17 This phenomenon needs to be substantiated in a systematic study. We aimed to retrospectively determine HPC rates on 120-kV and 190-keV images derived from DECT data and the magnitude of hematoma volume overestimation on follow-up 120-kV images in patients after admission contrast-enhanced WBCT.
Materials and Methods
This retrospective study was Health Insurance Portability and Accountability Act–compliant, and permission was obtained from University of Maryland, Baltimore institutional review board. Informed consent was waived. Consecutive series of patients referred to the trauma resuscitation unit were eligible. The inclusion criteria were the following: 1) a history of blunt trauma with acquisition of noncontrast head DECT followed by contrast-enhanced WBCT, as a part of diagnostic work-up of blunt polytrauma, between May 15, 2016, and September 10, 2016, with a confirmed diagnosis of hemorrhagic contusion on admission CT; 2) acquisition of follow-up noncontrast head DECT within 3 days of admission CT; and 3) 18 years of age or older. Patients were excluded if the mechanism of injury was penetrating trauma or if they underwent surgical interventions to address their contusions.
Subjects
A search of our radiology information system data base from the designated period yielded 171 patients with initial and at least 1 follow-up study performed on a DECT scanner. A radiologist with 11 years of experience reviewed the initial and follow-up DECT studies to select all the patients with hemorrhagic contusions (n = 40) that constituted the study group. The mean age of the final cohort was 38.4 years (range, 18–73 years), with 26 men and 14 women.
Reference Standard
HPC is defined as an enlargement of ≥30% of the original hematoma volume on follow-up CT studies as suggested by Alahmadi et al.18
Imaging Technique
Admission WBCT and follow-up head examinations were performed on a DECT scanner (Somatom Force; Siemens, Erlangen, Germany). WBCT involves a noncontrast head CT followed by contrast-enhanced CT of the neck, chest, abdomen, and pelvis. The studies were performed after injection of 100 mL of iodinated contrast media (iohexol, Omnipaque 300; GE Healthcare, Piscataway, New Jersey) using a biphasic injection with 60 mL injected at 5 mL/s and 40 mL injected at 4 mL/s. The contrast injection was followed by a 50-mL saline injection at 4 mL/s.
DECT head images were obtained with the x-ray tubes at 80 kV and Sn150kV (150 kV+ tin filter). Scan parameters were as follows: rotation time, 0.5 seconds; pitch, 0.55. The reference milliampere-second was 273 for the Sn150kV and 410 for the 80-kV tube. Original dual-energy datasets were reconstructed with an increment of 1 mm and a slice thickness of 1 mm. Automatic reconstruction of 120-kV-equivalent mixed-DECT images at 5-mm slice thickness and 5-mm intervals using an adaptive iterative reconstruction algorithm (ADMIRE, Siemens) with a strength value of 3 was performed and sent to the PACS at the time of the study. Automatic tube current modulation (CARE Dose4D; Siemens) was used in all patients.
Image Analysis of DECT
DECT data from admission and follow-up head CTs were processed to derive 190-keV image sets at 5-mm slice thickness and 5-mm intervals on a workstation (syngo.via, Version VB10B; Siemens) and sent to the PACS. Both 120-kV and 190-keV image sets from the PACS (5-mm slice thickness, 5-mm intervals) were loaded onto the thin-client server at our institution (IntelliSpace Portal; Philips Healthcare, Best, the Netherlands) to facilitate hematoma volume measurements. Hence, 4 image sets were used for volume measurements in each patient (ie, 120-kV, 190-keV image sets [from the admission study] and 120-kV, 190-keV [from the follow-up study]). Reviewer 1 annotated the hemorrhagic contusions that were meant for volume measurements. In patients with multiple contusions, the largest lesion was used for measurement. Reviewer 2 (third-year radiology resident) measured the volumes of the hemorrhagic contusions in each patient. Measurements were randomly performed regarding patient order and the order of the image sets to avoid potential preconceived bias. Volumes were measured using semiautomated 3D segmentation. An ROI was drawn on axial slices with the use of coronal and sagittal slices to exclude unwanted surrounding brain tissue from the ROIs (Fig 1D–F).19
Statistical Analysis
Statistical analysis was performed by K.S. using statistical software (JMP 12 software; SAS Institute, Cary, North Carolina). Contingency analysis was used to compare dichotomous variables. The McNemar test was used to compare the frequencies of HPC on 120-kV and 190-keV images. For comparison of hematoma volumes obtained by 120-kV and 190-keV, Bland-Altman analysis was performed. To determine the correlation of volume measurements on both image sets, we calculated the Pearson correlation coefficient. The Wilcoxon signed rank test was used to test the potential differences between the median values obtained by both methods. Regression analysis was performed to identify the best predictor of the percentage of hematoma volume overestimation. The following formulas were used to calculate the different variables: Percentage of Hematoma Overestimation = 100 (Volume on Follow-Up 120-kV − Volume on Follow-Up 190-keV) / (Volume on Follow-Up 190-keV); Percentage of Hemorrhagic Progression on 190 keV = 100 (Volume of Hematoma on Follow-Up 190-keV − Volume on Admission 190-keV) / (Volume on Admission 190-keV); similarly, the percentage of hemorrhagic progression on 120 kV = 100 (Volume of Hematoma on Follow-Up 120-kV − Volume on Admission 120-kV) / (Volume on Admission 120-kV). A P value of < .05 was considered significant.
Results
The median time to follow-up noncontrast head CT was 6 hours (quartile 3 − quartile 1 = 4.75 hours) after admission contrast-enhanced WBCT. On follow-up 120-kV images, HPC was detected in 24 of the 40 patients (60%), with HPC defined as an enlargement of ≥30% of the original hematoma volume. On 190-keV images, HPC was detected in only 17 of the 40 patients (43%). The McNemar test showed that HPC was more frequently observed on 120-kV images with a test result of 7 (P = .008).
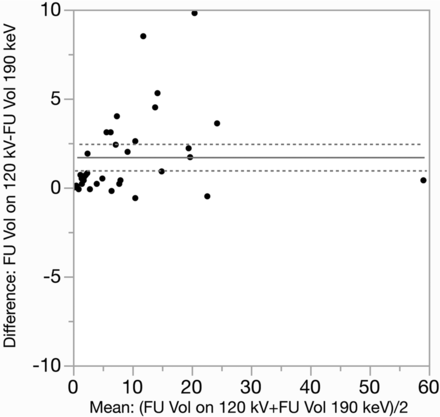
Bland-Altman analysis showed that hematoma volumes were systematically overestimated on follow-up 120-kV images (9.68 versus 8 mm3; mean difference, 1.68 mm3; standard error, 0.37; P < .001) compared with 190-keV images (Fig 2). There was no significant difference in the measured volumes between admission 120-kV and 190-keV images (6.11 versus 6.07 mm3; mean difference, 0.045 mm3; standard error, 0.05; P = .22). The Pearson correlation coefficient for hematoma volumes determined by 120-kV and 190-keV was r = 0.999 (P < .001) for admission head CTs and r = 0.98 (P < .001) for follow-up head CTs. The mean percentage of hematoma volume overestimation on follow-up 120-kV images was 29% ± 32% (95% CI, 18%–39%), and the median was 22% (quartile 3 − quartile 1 = 36.8).
Bland-Altman plots of the hematoma volume overestimation on follow-up 120-kV images compared with 190-keV images. Horizontal lines represent the mean volume overestimation (solid line) and limits of agreement (dashed lines). FU indicates follow-up; Vol, volume.
Regression analysis was performed using the hematoma volume on admission CT (190-keV), hematoma volume on follow-up CT (190-keV), time to follow-up, absolute volume of hemorrhagic progression (190-keV), and percentage of hemorrhagic progression as independent variables with percentage of hematoma overestimation as a dependent variable. The percentage of HPC was the best predictor of the percentage of hematoma volume overestimation (β = 16.4; 95% CI, 9.91–22.97; standard error, 3.2; P < .001).
Discussion
The major findings from our study in patients after admission contrast-enhanced WBCT are the following: 1) follow-up 120-kV head CT images, which are akin to SECT images, significantly overestimate the hematoma volume and hence the rate of HPC, 2) mean and median percentages of hematoma volume overestimations were 29% and 22%, respectively, and 3) the best predictor of percentage of volume overestimation was the percentage of hemorrhagic progression on follow-up CT (ie, the magnitude of overestimation is proportional to the magnitude of HPC).
Our study demonstrated that the true occurrence of HPC in patients after contrast-enhanced WBCT, as measured from the 190-keV images, was 43%. This number is similar to the 45% that was reported in conservatively managed patients by Alahmadi et al,18 who had similar selection criteria and cutoff values for progression. However, the rate increased to 60% if measured on 120-kV images (images comparable with SECT images) because there were 7 patients (18%) who were wrongly diagnosed with HPC. This higher rate of HPC is caused by “pseudohematoma.” We describe pseudohematoma as the volume of nonhemorrhagic hyperattenuating brain parenchyma caused by iodinated contrast leak from the permeable capillary endothelium. The leaked contrast from the bolus administered during the admission WBCT is retained in the brain parenchyma and would be seen in the follow-up studies as an area of hyperattenuation on 120-kV images, simulating a hematoma. The exact duration of this parenchymal contrast retention has not yet been analyzed and reported.
The attenuation contribution of iodine is negligible at the higher end of the monochromatic energy spectrum.13,16,17 Hence, 190-keV images demonstrate the actual hematoma size by displaying attenuation contributed predominantly by hematoma. It was recently demonstrated that virtual noncontrast images are comparable with 190-keV images in minimizing the attenuation contribution of iodine to negligible levels.13 Hence, virtual noncontrast images, if used in place of 190-keV images, may have yielded similar results in obtaining true hematoma volumes. Enhancement of cerebral contusion on contrast-enhanced SECT was described by Huang et al20; however, the authors did not describe how they were able to differentiate the hyperattenuation caused by iodinated contrast from that of the hematoma.
Cerebral contusions can be divided into 3 distinct regions: 1) epicenter, 2) penumbra, and 3) parapenumbra.2 The epicenter receives the peak energy from impact, and surrounding regions receive progressively less energy with distance. Energy deposited in the epicenter is sufficient to fracture capillaries, resulting in an immediate hemorrhagic lesion. In the penumbra and parapenumbra, the energy is not enough to fracture the capillaries but activates the mechanosensitive molecular processes that will lead to the delayed structural failure of capillaries.21,22 Animal studies have shown that the mechanosensitive molecular process begins with transcriptional up-regulation, followed by opening of the sulfonylurea receptor 1 (SUR1) SUR1-regulated channel.2,21 Opening the channel has been linked to gradual oncotic cell swelling and death of the endothelial cells, resulting in increased capillary permeability, and progression to capillary fragmentation. Increased permeability results in vasogenic edema, while capillary fragmentation results in extravasation of blood, contributing to HPC. HPC rates are directly related to the degree of endothelial damage and capillary fragmentation. The penumbra and parapenumbra usually manifest as vasogenic edema on CT because of increased permeability of the damaged endothelium.2 However, after contrast administration, enhancement of the parenchyma in the penumbra and parapenumbra results from the leak of iodinated agents through the same damaged endothelium, resulting in pseudohematoma.20 The phenomenon of HPC rates being proportional to the degree of capillary disruption caused by the mechanosensitive molecular process likely explains our other finding (ie, the magnitude of overestimation of hematoma is proportional to the magnitude of HPC because both are directly related to the degree of endothelial damage and capillary fragmentation).
Several authors have reported HPC as the reason for an operation, sometimes in up to 20% of patients.3⇓⇓⇓⇓–8 Studies have also reported that patients with progression of injury on a repeat CT underwent more frequent interventions related to intracranial pressure management changes, ventriculostomy placement or adjustment, addition of antiseizure medication, repeat CT, intensive care unit observation, delay in extubation, and initiation of thromboprophylaxis, even in patients with stable or improved Glasgow Coma Scale scores.12,18,23⇓–25 Similarly, Alahmadi et al18 reported a significant association between radiographic progression of contusions and the need for neurosurgical interventions, even though the mean Glasgow Coma Scale score and mean change in Glasgow Coma Scale score during the hospital course showed no difference.18 Hence, an accurate assessment of HPC has important clinical implications in preventing unnecessary interventions and radiation from follow-up imaging.
Using strict criteria in describing HPC, including indicating whether there was prior contrast administration, and detailing image sets used for measurement (SECT, 120 kV, or 190 keV) would minimize potential errors in identifying contusion progression. Health care providers should account for overestimation of hematoma volumes if the follow-up studies were performed on SECT. We also suggest that future research articles describe in detail the imaging techniques in their methods section, because contrast-enhanced WBCT has become a widely used technique for the work-up of the patient with blunt polytrauma.
Limitations
Our study has several limitations. It is a retrospective single-center design, which introduces selection and institutional bias. The cohort comprised only those patients who underwent WBCT studies at the time of admission. Thus, these data may not be generalizable to all trauma patients who may have undergone focused imaging of the head. A lack of consistent protocol for follow-up CTs (though most scans were obtained within 9 hours) may have affected the values. Finally, we excluded patients who underwent neurosurgical interventions. Those patients may differ in the severity of endothelial damage and hence have a different magnitude of iodine leak and hematoma volume overestimation.
Conclusions
Mixed 120-kV images from DECT and by extension the SECT images overestimate hematoma volumes, hence the HPC rates on follow-up head CTs, in patients after contrast-enhanced WBCT. Our study demonstrates that it is important to perform follow-up of cerebral contusions on a DECT scanner and use high-monochromatic (190-keV) images in patients after contrast-enhanced WBCT to accurately estimate HPC.
Footnotes
Disclosures: Uttam K. Bodanapally—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Siemens, Comments: talk on the role of “Dual-Energy CT in Patients with Traumatic Brain Injury” at the American Society of Head and Neck Radiology Annual Meeting in Las Vegas, September 17, 2017; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: Siemens, Comments: talk on the role of “Dual-Energy CT in Patients with Traumatic Brain Injury” at American Society of Head and Neck Radiology Annual Meeting in Las Vegas, September 17, 2017. David Dreizin—UNRELATED: Grants/Grants Pending: Radiological Society of North American research scholar grant, Siemens.* *Money paid to the institution.
References
- Received September 8, 2017.
- Accepted after revision December 11, 2017.
- © 2018 by American Journal of Neuroradiology