Abstract
BACKGROUND AND PURPOSE: Glutathione is an important antioxidant in the human brain and therefore of interest in neurodegenerative disorders. The purpose of this study was to investigate the feasibility of measuring glutathione in healthy nonsedated children by using the 1H Mescher-Garwood point-resolved spectroscopy (MEGA-PRESS) sequence at 3T and to compare glutathione levels between the medial parietal gray matter and the cerebellum.
MATERIALS AND METHODS: Glutathione was measured using MEGA-PRESS MRS (TR = 1.8 seconds, TE = 131 ms) in the parietal gray matter (35 × 25 × 20 mm3) of 6 healthy children (10.0 ± 2.4 years of age; range, 7–14 years; 3 males) and in the cerebellum of 11 healthy children (12.0 ± 2.7 years of age; range, 7–16 years; 6 males). A postprocessing pipeline was developed to account for frequency and phase variations in the edited ON and nonedited OFF spectra. Metabolites were quantified with LCModel and reported both as ratios and water-scaled values. Glutathione was quantified in the ON-OFF spectra, whereas total NAA, total Cho, total Cr, mIns, Glx, and taurine were quantified in the OFF spectra.
RESULTS: We found significantly higher glutathione, total Cho, total Cr, mIns, and taurine in the cerebellum (P < .01). Glx and total NAA were significantly higher in the parietal gray matter (P < .01). There was no significant difference in glutathione/total Cr (P = .93) between parietal gray matter and cerebellum.
CONCLUSIONS: We demonstrated that glutathione measurement in nonsedated children is feasible. We found significantly higher glutathione in the cerebellum compared with the parietal gray matter. Metabolite differences between the parietal gray matter and cerebellum agree with published MRS data in adults.
ABBREVIATIONS:
- GSH
- glutathione
- MEGA-PRESS
- Mescher-Garwood point-resolved spectroscopy
- PGM
- parietal gray matter
- Tau
- taurine
- t-
- total
Glutathione (GSH) is an important antioxidant in the human brain1,2 and has been shown to be altered in a number of pathologies.3⇓⇓⇓–7 Measuring GSH is of interest in neurodegenerative disorders8 and may be a potential mechanistic biomarker for oxidative stress–related diseases and the efficacy of antioxidative treatments. Our specific interest is the quantification of GSH in the brains of children affected by neurodegenerative diseases such as ataxia telangiectasia, requiring a robust method to measure GSH levels in nonsedated children.
GSH can, in principle, be measured in vivo with 1H-MRS. However, due to the low concentration of GSH in the healthy human brain and in particular its overlap with higher concentration metabolites, quantification of GSH at 3T with conventional MRS sequences is controversial.9⇓–11 Spectral editing allows the removal of overlapping resonances for a direct and robust quantification of GSH.12⇓–14 While spectral editing can be used to specifically measure GSH, it is more susceptible to subject motion than conventional single-voxel MRS sequences due to longer scan times and because spectral editing is a subtraction technique that relies on consistent data acquisition.
Few studies have reported in vivo GSH values in healthy children,15,16 and, to our knowledge, there is no study that specifically measured GSH in children using spectral editing techniques or that reported GSH in the cerebellum. Therefore, the goal of this study was to investigate the feasibility of measuring GSH in healthy nonsedated children with the Mescher-Garwood point-resolved spectroscopy (MEGA-PRESS)13,17 sequence and to compare GSH levels obtained in the medial parietal gray matter (PGM) with those measured in the cerebellum. To minimize subtraction errors caused by phase and frequency variations, we adapted a MEGA-PRESS postprocessing technique published by An et al17 and modified it to work with our data.
Materials and Methods
Subjects
Healthy children were recruited as part of an ongoing study, approved by the UK National Research Ethics Service East Midlands–Derby Committee (Reference 14/EM/1175). Informed consent was obtained from parents or guardians of participants. MRS data in the PGM were acquired in 6 children (10.0 ± 2.4 years of age; range, 7–14 years; 3 males). MR spectroscopic data in the cerebellum were acquired in 11 children (12.0 ± 2.7 years of age; range, 7–16 years; 6 males). There was no significant difference in age between the 2 groups (t test, P = .14). Structural MRI was checked by a neuroradiologist (R.A.D.) to ensure that children were neuroradiologically healthy.
Data Acquisition
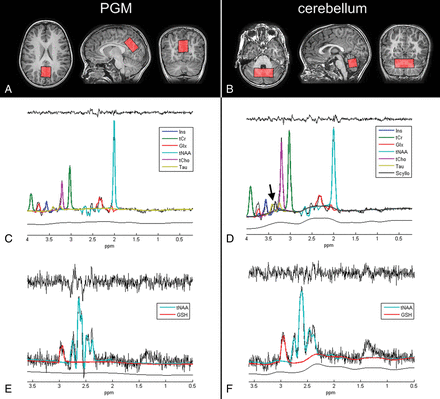
Data were acquired on a 3T MR scanner (Discovery MR750; GE Healthcare, Milwaukee, Wisconsin) equipped with a 32-channel head coil, without sedation. In addition to standard pediatric MRI preparation, younger participants were shown an animation to help prepare them for the MRI,18 and participants could watch videos on an MRI–compatible monitor during the scan to improve tolerance. The MRI protocol included a 3D fast-spoiled gradient recalled T1-weighted structural MRI with 1-mm isotropic resolution for MRS planning (TR = 8.15 ms, TE = 3.172 ms, TI = 900 ms, FOV = 256 × 256 × 156 mm). Single-voxel MEGA-PRESS GSH editing was performed using TR = 2 seconds, TE = 131 ms, and 128 ON and 128 OFF acquisitions. Spectral editing was achieved with sinc-weighted Gaussian pulses with a pulse length of 20 ms (bandwidth, 64 Hz) applied at 7.5 ppm (OFF) and 4.54 ppm (ON) for editing GSH.13,17 A nonedited, water-unsuppressed reference scan of 16 averages was acquired at identical acquisition parameters. MRS voxel sizes were 35 × 25 × 20 mm in the PGM and 50 × 22 × 22 mm in the cerebellum. Typical voxel locations are illustrated in Fig 1. Total acquisition time for MEGA-PRESS MRS was 9 minutes 30 seconds.
Examples of the MRS voxel placement in the PGM (A) and cerebellum (B) in two 7-year-old children. Corresponding OFF (C and D) and ON-OFF (E and F) spectra are shown below. Individual metabolite spectra fitted by LCModel are highlighted in different colors. D, The arrow highlights visible resonances that are particularly large in the cerebellum and likely belong to taurine and scyllo-inositol. Residuals of the fits (black, upper row) and estimated LCModel baselines (black, lower row) are shown for each spectrum.
MRS Processing
MEGA-PRESS data were processed off-line with Matlab (MathWorks, Natick, Massachusetts). The workflow steps A–H are illustrated in On-line Fig 1. The phase angles between the 32 coil elements were calculated from the average unsuppressed water signal (A). All ON and OFF spectra were subsequently phased using the water phase angles and coil-combined by using the maximum peak height of the unsuppressed water signal as weighting factors (B). The resulting coil-combined 128 ON and 128 OFF spectra were potentially out of phase relative to each other due to subject motion and frequency drift. To correct for this, we phased individual ON and OFF spectra by maximizing the correlation of the NAA peak between the real and absolute part of the spectrum in the range of 1.87 and 2.21 ppm (C). Next, a reference ON spectrum for phase and frequency correction was created, like that described by An et al17 by pair-wise aligning the 128 ON spectra in the spectral range of 1.3 and 3.3 ppm and iterative pair-wise averaging of the spectra with the smallest root mean square error (D). All 128 ON and 128 OFF spectra were subsequently aligned to this ON reference spectrum by time domain phase and frequency correction using the fminsearch function (https://de.mathworks.com/help/matlab/ref/fminsearch.html) (E) in Matlab. For the alignment, the correlation coefficient of the real part of the NAA peak (1.87–2.21 ppm) between the ON reference spectrum and the individual ON and OFF spectra was maximized. The 128 aligned ON and OFF spectra were then averaged to 1 ON and 1 OFF spectrum each (F). Individual poor-quality ON and OFF spectra were detected by calculating the correlation coefficient of the NAA peak (1.87–2.21 ppm) between the 128 ON/OFF spectra and the averaged ON/OFF spectrum, respectively (G). In our data, a correlation coefficient threshold of 0.8 (1 indicating perfect correlation) was used to pair-wise exclude individual ON and OFF spectra. Finally, the new ON and OFF average spectra were recalculated (H) and subtracted to obtain the final edited ON-OFF spectrum.
Metabolite Quantification
Unedited MEGA-PRESS OFF spectra were analyzed between 4 and 0.2 ppm with LCModel (Version 6.3-1H; http://www.lcmodel.com/lcmodel.shtml)19 using a basis set simulated with TARQUIN (http://tarquin.sourceforge.net/).20 The OFF spectra were used to quantify total NAA (tNAA = NAA+ N-acetyl aspartylglutamate), total Creatine (tCr = Cr + phosphocreatine), total Choline (tCho = glycerophosphorylcholine + phosphorylcholine), mIns, Glx, and Taurine (Tau). The edited ON-OFF MEGA-PRESS spectra were analyzed in the range of 3.6–0.2 ppm with LCModel, using a measured basis set from GSH and NAA solutions. LCModel parameters for the ON-OFF analysis are given in the On-line Appendix.
Metabolite values are reported as ratios to tCr and as water-scaled values using the unsuppressed OFF water signal. For the latter, corresponding structural T1WI was segmented via SPM software (http://www.fil.ion.ucl.ac.uk/spm/software/spm12) into gray matter, white matter, and CSF and aligned with the MRS volume with in-house software. Water-scaled metabolite values were corrected for CSF contamination by dividing them by 1–CSF%. Because the complex anatomic structure in the cerebellum would not allow an accurate separation of gray and white matter, we did not take their differences in water content into account. T1 and T2 correction was not performed; thus, water-scaled and CSF-corrected metabolite values are reported as institutional units.
A 2-sample t test assuming unequal variances was used to determine significant differences in metabolite values and ratios between the PGM and the cerebellum.
Results
The proposed GSH processing gave robust results in all spectra. On-line Fig 2 shows the edited ON-OFF spectra and corresponding GSH fits determined by LCModel for all subjects. An example in On-line Fig 1 illustrates the correction for phase and frequency shifts in a PGM spectrum acquired in an 11-year-old girl.
Figure 1 shows sample spectra from the PGM and cerebellum and corresponding metabolite fits for two 7-year-old children. The OFF spectra from the cerebellum show visible resonances between 3.3 and 3.5 ppm (see the arrow in Fig 1D). LCModel assigned these resonances to taurine (around 3.4 ppm) and scyllo-inositol (around 3.34 ppm), but the fitted scyllo-inositol signals were too small for reliable quantification in both the cerebellum (Cramer-Rao lower bounds, 21% ± 22%) and PGM (Cramer-Rao lower bounds, 33% ± 27%). We therefore only report the apparent taurine signal for the cerebellum (Cramer-Rao lower bounds, 9% ± 1%) and PGM (Cramer-Rao lower bounds, 14% ± 2%). However, the combined sum of Tau + scyllo-inositol showed the same trends as taurine using either water scaling or ratios (results not shown).
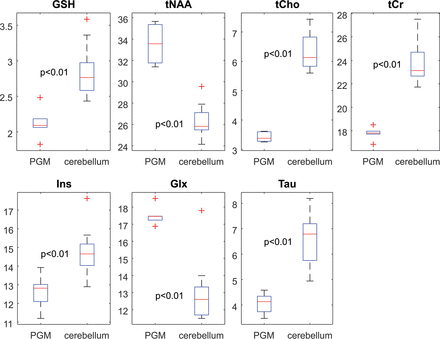
We found significantly higher GSH in the cerebellum compared with the PGM (P < .01). Additionally, we found higher tCho (P < .01), tCr (P < .01), mIns (P < .01), and Tau (P < .01) in the cerebellum, whereas NAA (P < .01) and Glx (P < .01) were significantly higher in the PGM. Boxplots are shown in Fig 2.
Boxplots showing the water-scaled metabolite values in institutional units in the PGM and cerebellum.
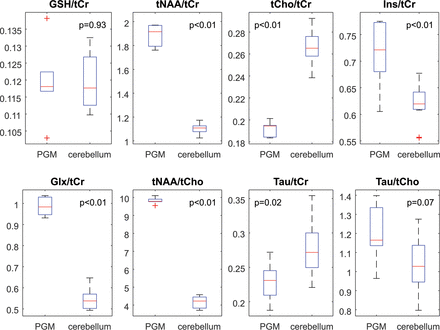
Figure 3 shows the results of the metabolite ratios between the PGM and cerebellum. There was no significant difference in GSH/tCr (P = .93). However, tNAA/tCr (P < .01), tNAA/tCho (P < .01), mIns/tCr (P = .01), and Glx/tCr (P < .01) were significantly higher in the PGM. Both tCho/tCr (P < .01) and Tau/tCr (P = .02) were lower in the PGM.
Boxplots showing the metabolite ratios in the PGM and cerebellum.
Discussion
We demonstrate reliable detection of glutathione levels in the parietal gray matter and cerebellum of nonsedated children using a dedicated proton MRS protocol for acquisition and postprocessing. Postprocessing of GSH MEGA-PRESS MRS was challenging, mainly because the editing pulse at 4.54 ppm also eliminates most of the residual water signal in the ON spectrum; this feature makes it difficult to precisely match its phase to the OFF spectrum. This difficulty can lead to subtraction artifacts, which are most often visible around the 2-ppm area and could severely bias GSH quantification due to residual overlapping Cr signal at 3 ppm. We therefore adapted and modified a previously published postprocessing approach.17 Whereas An et al17 focused on the complex spectral values from the Cr, Cho, and NAA peaks for alignment of individual spectra, our processing was simplified by focusing the alignment on the real part of the NAA and omitting zero and first-order baseline adjustments. This was performed to reduce the number of fitted parameters for a more robust parameter determination in our lower SNR spectra caused by a smaller voxel size.
To the best of our knowledge, this is the first study to compare GSH levels in the cerebrum with those found in the cerebellum in children. Few studies compared GSH levels between the cerebellum and the cerebrum in adults. In agreement with our findings, Emir et al21 found higher GSH and tCr in the vermis compared with the occipital cortex and posterior cingulate in healthy adults using a short-echo STEAM sequence at 7T, albeit without T1, T2, and CSF correction. An extensive postmortem study in humans by Tong et al22 showed no significant difference in GSH between the occipital and cerebellar cortices in the 1- to 18-year age group. A histologic study in mice by Kang et al23 revealed slightly lower GSH in the cerebellum compared with the cortex.
Previous studies reported higher Cho and Cr levels in the cerebellum compared with the cerebrum in healthy adults,24,25 in agreement with the results in our cohort of children, whereas an MR spectroscopic imaging study by Lecocq et al26 found only reduced NAA in the cerebellum. Looking at metabolite ratios, several studies found lower tNAA/tCr in the cerebellum compared with the cerebrum in children27 and adults,28⇓–30 in agreement with our findings. Additionally, Goryawala et al31 showed lower Glx/tCr in the cerebellum in adults, in agreement with our results in children. Another noteworthy finding in this study was higher apparent Tau/tCr in the cerebellum compared with the PGM. Taurine is particularly high in infants and children in the cerebellar cortex.32,33 Additionally, elevated taurine is characteristic of medulloblastomas,34 mainly originating in the cerebellum of children.
A limitation of this study is the relatively small sample size and the use of different subjects to scan the PGM and the cerebellum. Scanning the PGM and cerebellum in each subject would have allowed a pair-wise statistical analysis and likely reduced variability. We were, however, limited by time constraints in the MRI protocol due to the long acquisition time of GSH MEGA-PRESS MRS. Additionally, the MRS voxel in the cerebellum was relatively large, to ensure high-enough SNR for GSH quantification, thus having a relatively heterogeneous tissue composition, including the cerebellar gray matter (vermis and cortex) and the underlying cerebellar white matter.
Conclusions
We demonstrated that GSH measurement in nonsedated children is feasible. We found higher GSH in the cerebellum compared with the PGM. Differences in the other metabolites agree with published MRS data in adults; this finding suggests no major metabolic maturation effect from 7 years of age and older in our dataset.
Footnotes
Disclosures: Felix Raschke—RELATED: Grant: A-T Children's Project and Action for A-T, Comments: This work was supported by a research grant awarded by the 2 named charities*; UNRELATED: Support for Travel to Meetings for the Study or Other Purposes: Guarantors of Brain, Comments: travel grant to support travel to the International Society for Magnetic Resonance in Medicine meeting in 2015. Ralph Noeske—UNRELATED: Employment: GE Healthcare. Robert A. Dineen—RELATED: Grant: A-T Children's Project and Action for A-T.* Dorothee P. Auer—RELATED: Grant: A-T Children's Project and Action for A-T*; UNRELATED: Grants/Grants Pending: UK charities, UK research councils, National Institute for Health Research, UK funding.* *Money paid to the institution.
F.R. and the cost of the pediatric MRI scans were funded by the A-T Children's Project and Action for A-T.
References
- Received June 22, 2017.
- Accepted after revision September 13, 2017.
- © 2018 by American Journal of Neuroradiology