Abstract
BACKGROUND AND PURPOSE: Signal intensity increases possibly suggestive of gadolinium retention have recently been reported on unenhanced T1-weighted images of the pediatric brain following multiple exposures to gadolinium-based MR contrast agents. Our aim was to determine whether T1 signal changes suggestive of gadolinium deposition occur in the brains of pediatric nonneurologic patients after multiple exposures to gadobenate dimeglumine.
MATERIALS AND METHODS: Thirty-four nonneurologic patients (group 1; 17 males/17 females; mean age, 7.18 years) who received between 5 and 15 injections (mean, 7.8 injections) of 0.05 mmol/kg of gadobenate during a mean of 2.24 years were compared with 24 control patients (group 2; 16 males/8 females; mean age, 8.78 years) who had never received gadolinium-based contrast agents. Exposure to gadobenate was for diagnosis and therapy monitoring. Five blinded readers independently determined the signal intensity at ROIs in the dentate nucleus, globus pallidus, pons, and thalamus on unenhanced T1-weighted spin-echo images from both groups. Unpaired t tests were used to compare signal-intensity values and dentate nucleus–pons and globus pallidus–thalamus signal-intensity ratios between groups 1 and 2.
RESULTS: Mean signal-intensity values in the dentate nucleus, globus pallidus, pons, and thalamus of gadobenate-exposed patients ranged from 366.4 to 389.2, 360.5 to 392.9, 370.5 to 374.9, and 356.9 to 371.0, respectively. Corresponding values in gadolinium-based contrast agent–naïve subjects were not significantly different (P > .05). Similarly, no significant differences were noted by any reader for comparisons of the dentate nucleus–pons signal-intensity ratios. One reader noted a difference in the mean globus pallidus–thalamus signal-intensity ratios (1.06 ± 0.006 versus 1.02 ± 0.009, P = .002), but this reflected nonsignificantly higher T1 signal in the thalamus of control subjects. The number of exposures and the interval between the first and last exposures did not influence signal-intensity values.
CONCLUSIONS: Signal-intensity increases potentially indicative of gadolinium deposition are not seen in pediatric nonneurologic patients after multiple exposures to low-dose gadobenate.
ABBREVIATIONS:
- DN
- dentate nucleus
- GBCA
- gadolinium-based contrast agent
- Gd
- gadolinium
- GP
- globus pallidus
- NSF
- nephrogenic systemic fibrosis
- SI
- signal intensity
Recent reports have detailed high signal intensity (SI) in certain brain areas (primarily the dentate nucleus [DN] and globus pallidus [GP]) on unenhanced T1-weighted images following multiple exposures to gadolinium-based contrast agents (GBCAs).1⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓⇓–20 Many of these reports have focused on apparent differences between macrocyclic and open-chain “linear” GBCAs,4⇓⇓⇓⇓⇓⇓⇓⇓–13 invariably associating progressive T1 hyperintensity with multiple exposures to linear GBCAs and concluding that observed T1 signal reflects the lower stability of these agents and thus a greater propensity for gadolinium (Gd) release and, subsequently, deposition in the brain. Among the more recent reports are several that describe retrospective assessments in pediatric patients.15⇓⇓⇓–19 Although each patient evaluated received just 1 specific linear GBCA (gadopentetate dimeglumine; Magnevist; Bayer HealthCare, Wayne, New Jersey), the study-based recommendations in each case were to consider carefully the use of all linear agents in pediatric subjects.
Gadobenate dimeglumine (MultiHance; Bracco Diagnostics, Monroe, New Jersey) is an ionic open-chain, linear GBCA that differs fundamentally from gadopentetate and other extracellular GBCAs in having an aromatic substituent on the chelating molecule.21 Unique properties conferred by this substituent include increased R1-relaxivity,22 which permits the acquisition of diagnostically valid images with a reduced dose,23 and liver-specificity, which permits gadobenate use for hepatobiliary-phase liver applications.24 An additional benefit is increased molecular stability compared with gadopentetate, other linear agents, and certain macrocyclic agents.25 Studies that have evaluated brain T1 signal intensities after multiple exposures to gadobenate have yielded conflicting results with one report demonstrating T1 signal increases, albeit to a lesser extent than with gadopentetate,10 and others demonstrating no direct changes.11,12
We aimed to determine whether multiple exposures to low-dose gadobenate for nonneurologic pathology results in T1 signal changes in the DN and GP of pediatric patients relative to that in age- and weight-matched GBCA-naïve control subjects.
Materials and Methods
Participants
This single-center (Saarland University Medical Center, Homburg/Saar, Germany) prospective study was approved by the institutional review board. Written informed consent for the use of imaging data was obtained routinely from all parents or legal guardians at the time of the first examination. Pediatric (younger than 18 years of age), mainly oncologic patients referred for diagnosis and subsequent therapy monitoring who had undergone at least 5 MR imaging examinations enhanced solely with gadobenate and who had no known neurologic disease or symptoms were identified from our electronic data base. Initial exposure to the first patient meeting these criteria was in August 2008. Patients with unsatisfactory images because of motion artifacts, an unknown history of GBCA administration, or severely impaired renal function (glomerular filtration rate < 30 mL/min) were excluded. All eligible patients underwent unenhanced T1-weighted imaging of the brain as a prospective adjunctive acquisition immediately before the next scheduled routine follow-up examination (performed in all cases between September 2015 and March 2016). Thirty-four patients met the inclusion criteria (group 1). A further 24 age- and weight-matched subjects with no known neurologic disease or symptoms who had never been administered any GBCA composed a GBCA-naïve control group (group 2). Patients in group 1 received 5 (n = 9), 6 (n = 7), 7 (n = 3), 8 (n = 4), 9 (n = 2), 10 (n = 3), 11 (n = 1), 12 (n = 2), 13 (n = 1), 14 (n = 1), and 15 (n = 1) injections of gadobenate each at a dose of 0.05 mmol/kg of body weight (0.1 mL/kg of body weight).
Imaging Protocol
All brain MR imaging examinations were performed on 1.5T whole-body MR imaging systems (Magnetom Aera or Magnetom Symphony, Siemens, Erlangen, Germany). All examinations used an unenhanced axial T1-weighted spin-echo sequence (TR/TE, 450–650/7–12 ms; section thickness, 5 mm with a 1.5-mm gap). Unenhanced axial T2-weighted images were acquired with TR/TE, 4000–5400/80–100 ms; section thickness, 5 mm; FOV, 230 mm).
Data Collection
Four general consultant radiologists (A.B., P.R., G.R., and P.A., with 25, 4, 20, and 3 years' experience, respectively) and 2 neuroradiologists in consensus (C.C., M.M., with 35 and 5 years' experience, respectively; considered reader 5), who were all blinded to patient diagnoses and details of all contrast administrations, determined SI values in operator-defined oval ROIs positioned within the DN, GP, thalamus, and pons of all patients and control subjects, as described by Kanda et al.1 Each reader was instructed to make ROIs as large as possible (mean size, 10 mm2; range, 6–18 mm2). ROIs in the DN were placed on the right side whenever possible and were positioned as far as possible from pulsating vessels (if present), without including rim aspects. ROIs in the GP were placed in the capsula interna; ROIs in the central pons and thalamus were adjusted as appropriate to ensure homogeneity. If T1-weighted images were considered inconclusive for visualization, T2-weighted images were available to each reader for correlation, with ROIs then placed on the corresponding T1-weighted image.
Image sets for gadobenate-exposed and GBCA-naïve subjects were randomized, transcribed to a CD-ROM, and sent by mail to each blinded reader for viewing and independent evaluation on each reader's personal PACS workstation.
Statistical Analysis
Comparison of demographic characteristics between groups 1 and 2 was performed by using a Student t test for age and weight and a Fisher exact test for sex. The primary outcome measure was whether repeat exposure to gadobenate (group 1) resulted in statistically significant increases in brain intraparenchymal SI relative to that in age- and weight-matched GBCA-naïve control subjects (group 2). To evaluate the primary outcome measure, we calculated DN-pons and GP-thalamus SI ratios for all subjects. SI values determined in the DN, GP, thalamus, and pons as well as DN-pons and GP-thalamus SI ratios were compared between groups 1 and 2 with unpaired t tests. Differences were considered significant for P < .05. Generalized multivariate linear regression was used to determine whether SI ratios were influenced by the number of gadobenate injections (control group considered as zero injections), age, sex, or weight. The interreader reliability of SI measurements was determined from the intraclass correlation coefficient, obtained from the generalized random effects regression model.
Results
Demographic details of patients in groups 1 and 2 are presented in Table 1. The gadobenate-exposed subjects included 6 infants (2 years of age or younger at first exposure), 11 subjects from 2 to 8 years of age at first exposure, and 17 subjects 9 years of age or older at first exposure. No significant differences were noted for age, weight, or sex distribution between groups 1 and 2. Each patient in group 1 was administered MultiHance at a dose of 0.05 mmol/kg of body weight. Given that 1 mL of MultiHance solution for injection contains 334 mg of gadobenate,26 this corresponds to 33.4 mg of gadobenate/kg of body weight. On the basis of patient weight at each examination, a mean total accumulated dose of 9.8 ± 8.33 g of gadobenate (range, 1.67–37.41 g) was administered per patient at a mean volume of 3.85 ± 3.14 mL/examination (1.27 ± 1.05 g gadobenate/examination). Patient diagnoses and reasons for follow-up imaging of patients in group 1 are given in Table 2.
Summary of group characteristicsa
Summary of patient diagnosesa
SI values determined in the DN, GP, pons, and thalamus as well as the DN-pons and GP-thalamus SI ratios are reported in Table 3. No significant differences (P > .05) in T1 signal intensity between gadobenate-exposed and GBCA-naïve control subjects were noted in any brain region by any reader. Similarly, no significant differences between gadobenate-exposed and GBCA-naïve control subjects were noted by any reader for DN-pons SI ratios. A significant difference in GP-thalamus SI ratios was noted by 1 of 5 readers (reader 5), but this was considered anomalous due in part to nonsignificantly higher mean T1 signal intensity in the thalamus of control subjects. Generalized multivariate linear regression confirmed no influence of the number of gadobenate exposures on SI ratios across readers after adjusting for age, sex, and weight. Strong agreement among all readers was noted for SI assessments in the DN, GP, pons, and thalamus with intraclass correlation coefficient values ranging from 0.84 to 0.97 for gadobenate-exposed subjects and from 0.86 to 0.92 for GBCA-naïve control subjects.
Comparison of brain SI values and DN-pons and GP-thalamus SI ratios between gadobenate-exposed and GBCA-naïve control subjectsa
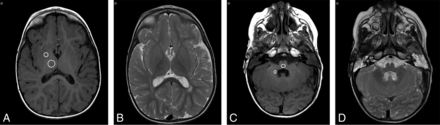
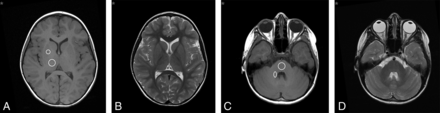
Figures 1 and 2 show representative T1- and T2-weighted images of pediatric brain regions after multiple injections of 0.05 mmol/kg of gadobenate in patients younger than 2 years of age (8 injections; total, 3.34 g of gadobenate) and ∼7 years of age (14 injections; total, 27.39 g of gadobenate), respectively.
Oncologic male patient (1 year 9 months of age at his first visit) undergoing follow-up imaging for cervical neuroblastoma in remission. Unenhanced T1- and T2-weighted transverse images of the DN-pons (A and B) and GP-thalamus (C and D) after 8 injections of 0.05 mmol/kg of gadobenate reveal no evidence of SI changes.
Oncologic female patient (7 years 2 months of age at her first visit) undergoing follow-up imaging for non-Hodgkin lymphoma in remission. Unenhanced T1- and T2-weighted transverse images of the DN-pons (A and B) and GP-thalamus (C and D) after 14 injections of 0.05 mmol/kg of gadobenate reveal no evidence of SI changes.
Discussion
To date, no clinical signs or symptoms associated with T1 signal increases in the brain have been reported and no consequences for patient health, including neurologic function, have been identified. Nevertheless, the latest version of the American College of Radiology Manual on Contrast Media27 recommends careful consideration of the clinical benefit versus the unknown potential risk of Gd deposition when deciding to perform a Gd-enhanced MR imaging study and particular attention paid to pediatric and other patients who may receive many GBCA-enhanced MR imaging studies during their lifetime. It further recommends taking into account multiple factors when selecting a GBCA, including diagnostic efficacy, relaxivity, rate of adverse reactions, dosing/concentration, and propensity to deposit in more sensitive organs such as the brain. Our findings in 34 nonneurologic pediatric patients who received between 5 and 15 administrations of low-dose (0.05 mmol/kg of body weight) gadobenate revealed no differences in T1 signal intensity in the DN, GP, pons, and thalamus relative to the SI measurements in 24 age- and weight-matched control subjects who had never been exposed to GBCA. Likewise, no significant differences in DN-pons SI ratios were noted, while just 1 of 5 blinded readers reported a significantly higher GP-thalamus SI ratio, which could be ascribed to higher T1 signal intensity in the thalamus of control subjects.
Our findings are in contrast to those of Flood et al,18 who found an increased DN-pons SI ratio in patients exposed to gadopentetate relative to GBCA-naïve control subjects (though no differences in GP-thalamus SI ratio were found) and in contrast to those of Hu et al,19 who found significantly higher T1 signal in the DN and GP of patients after serial exposure to gadopentetate than in the DN and GP of control subjects.
There are at least 2 possible reasons for the different findings. First, our patient population was different. Whereas Flood et al18 and Hu et al19 evaluated patients who had undergone multiple brain examinations, our patient cohort comprised mainly oncologic patients with no known brain abnormalities. It is thus possible that the patients evaluated by Flood et al and Hu et al were more prone to brain Gd deposition due to a more compromised blood-brain barrier. On the other hand, studies have demonstrated T1-signal increases in the DN and GP even in the presence of a seemingly intact BBB,14,28 implying that the potential for T1-hyperintensity may be independent of the patient's clinical status and dependent solely on the amount of Gd administered. However, most of the Gd found deposited in the brain is actually in the perivascular space and has not passed the BBB,29,30 while Gd that does appear to have crossed an intact BBB is found primarily in the neural tissue interstitium rather than in neural cells.14
Second, whereas gadobenate and gadopentetate are both ionic open-chain GBCAs, they differ fundamentally in that an aromatic substituent is present on the gadobenate molecule. On the one hand, this substituent influences the elimination profile of gadobenate, facilitating its excretion in part (typically up to 5% of the injected dose in subjects with normal renal function) via the hepatobiliary route.31 More pertinently, it also leads to markedly higher R1-relaxivity in vivo,22 which, in turn, leads to markedly increased SI enhancement on T1-weighted images for equivalent administered doses. A proven benefit of this higher relaxivity is that a lower gadobenate dose can be used to obtain diagnostically valid images.23,24 In our study, the mean accumulated dose across all patients was 9.8 ± 8.33 g, administered across a mean of 7.8 ± 2.9 examinations (mean, 3.85 ± 3.14 mL of MultiHance/examination, corresponding to 1.27 ± 1.05 g of gadobenate/examination). This is considerably lower than the mean accumulated gadopentetate dose of 16.2 ± 10.1 g across a mean of 5.9 ± 2.7 examinations (mean, 2.75 g gadopentetate/examination) given by Flood et al18 and would similarly be lower than that administered by Hu et al,19 given that they injected a standard dose of 0.1 mmol/kg of gadopentetate/examination. At our institution, we routinely administer 0.05 mmol/kg of gadobenate for pediatric oncologic imaging.
An additional feature conferred by the aromatic substituent, which is invariably overlooked when simplistic comparisons are made between “linear” and macrocyclic GBCAs,4⇓⇓⇓⇓⇓–10,32 is improved steric hindrance and thus increased kinetic inertia. An increased steric effect conferred by a bulky substituent potentially hinders unwrapping of the ligand around the gadolinium, thereby increasing molecular stability.33 Improved kinetic inertia due to an aromatic substituent has previously been demonstrated for gadofosveset.34 Unfortunately, accurate in vivo measurements of the kinetic stability of gadobenate are currently lacking, though its thermodynamic and conditional stability constants in vitro are the highest of all open-chain GBCAs and higher also than the macrocyclic agent gadobutrol.25 Further evidence of the inherent stability of gadobenate comes from the fact that this GBCA, unlike all other GBCAs except gadoterate meglumine (Dotarem; Guerbet, Aulnay-sous-Bois, France), does not contain any excess chelating agent in its formulation.25 Excess chelating agent is an indirect indicator of the potential of a GBCA to release Gd; that the gadobenate formulation does not contain excess chelate implies that it does not release Gd to the same extent as other GBCAs.
Our findings are in stark contrast to those of Weberling et al,10 who found significantly increased DN-pons and DN-CSF SI ratios after serial exposures of gadobenate to adults. However, several factors should be borne in mind when evaluating the results of Weberling et al. First, the patients evaluated were only required to have had a minimum of 5 consecutive gadobenate-enhanced MR imaging scans; each patient's first included scan was not necessarily the first scan the patient received, and prior scans with other GBCAs might have been performed. The likelihood that patients might have had prior MR imaging exams with other GBCAs is clearly a major confounding factor. Ramalho et al12 showed that significant T1 hyperintensity in deep brain nuclei occurred after the use of gadobenate in patients who had received prior administration of gadodiamide (Omniscan; GE Healthcare, Piscataway, New Jersey), but not in patients who had not previously received gadodiamide. An earlier report by Ramalho et al11 demonstrated significant SI increases in the DN and GP of patients who had received gadodiamide but not in patients who had received gadobenate. The lack of adequate patient screening by Weberling et al10 means that possible potentiating effects of prior exposure to other GBCAs could not be excluded.
Second, each patient evaluated by Weberling et al10 received a standardized volume of 15 or 20 mL of gadobenate per examination irrespective of body weight, resulting in a mean accumulated volume of 136.9 ± 57.6 mL (ie, a mean accumulated dose of 45.72 g of gadobenate) between the first and last MR imaging examinations. Given that an approved gadobenate dose for all indications other than the liver, kidneys, urinary tract, and adrenal glands is 0.1 mmol/kg of body weight,26 which corresponds to 15 mL for a 75-kg patient, any patient below 75 kg in weight would have received more than the approved dose (ie, more than the approved amount of Gd). Third, all except 1 of the patients evaluated by Weberling et al had brain metastases from melanoma, with many undergoing radiation therapy. These patients would certainly have had a more severely compromised BBB, potentially allowing easier GBCA access to deep brain nuclei.
Weberling et al10 ascribed their observed SI increases to Gd retention, speculating that their findings reflect the specific potential of gadobenate to release Gd. In drawing parallels with the postulated causative role of GBCAs in nephrogenic systemic fibrosis (NSF), they noted that gadobenate is classified as being of intermediate risk for NSF by the European Medicines Agency35; and in referring to 1 in vitro determination of kinetic stability conducted by a competitor to the manufacturer of gadobenate,36 they suggested that the potential for Gd release is similar for gadobenate and gadopentetate. Unfortunately, in vitro determinations of kinetic stability are inherently limited in that they cannot replicate normal physiologic conditions in vivo and cannot account for differing and unique routes of elimination among GBCAs. Although Weberling et al10 acknowledged that no unconfounded cases of NSF have been reported for gadobenate,37 they failed to point out that other relevant regulatory authorities, including the US Food and Drug Administration, classify gadobenate as having a low risk of NSF.38,39 In this regard, most (73%) of the unconfounded published NSF cases were reported in the United States,40 and at the height of the NSF crisis (2006–2010), just 1 macrocyclic GBCA (gadoteridol, ProHance; Bracco Diagnostics) had FDA approval for commercial use. Gadobutrol (Gadavist; Bayer Healthcare) and gadoterate (Guerbet) were approved in 2011 and 2013, respectively. The other approved GBCAs besides gadobenate were gadodiamide (GE Healthcare), gadoversetamide (OptiMARK; Covidien), and gadopentetate (Bayer Healthcare), which were avoided and subsequently contraindicated in patients with severe renal impairment,38 leaving only gadobenate as an approved open-chain GBCA for routine applications in this population.
If, as is widely accepted, NSF occurs because of Gd release from the chelating molecule,35 then at least 1 unconfounded case might have been expected for gadobenate if, as postulated by Weberling et al,10 Gd is released in vivo to an extent similar to that seen with gadopentetate. That no unconfounded cases have been reported despite exhaustive investigation41⇓–43 suggests that Gd is not released from gadobenate to the same extent and that any observed T1 hyperintensity reflects retention of the intact molecule. Roberts et al44 recently reported high levels of Gd in the skin of a patient who underwent 61 enhanced brain examinations with a variety of GBCAs and that speciation analysis revealed intact gadobenate.
Further studies are required to determine whether T1 hyperintensity is seen in some patients after serial gadobenate exposure, and if so, whether this reflects accumulation of intact gadobenate or released Gd bound to macromolecules (eg, neuromelanin). In this regard, it is possible that Gd released from less stable GBCAs binds to macromolecules and that the observed T1 hyperintensity reflects elevated T1-signal due to slowing of the molecular tumbling rate of these Gd-macromolecule complexes.30 This hypothesis might explain why elevated T1 signal is observed with less stable GBCAs despite the very small Gd concentrations shown to be retained20 and, conceivably, why detectable high signal is less evident with more stable GBCAs if these are retained as fully intact molecules rather than as Gd-macromolecular complexes.45 Of note, however, is the study by Stojanov13 and a recent study in pediatric subjects by Rossi Espagnet et al.46 that demonstrate quantifiable T1 signal increases after multiple exposures to the macrocyclic GBCAs gadobutrol and gadoterate, respectively.
Also worthy of study is the possible differential impact of GBCA R1-relaxivity on T1 hyperintensity if GBCAs are retained as intact molecules; it is likely that retained GBCAs that have low R1-relaxivity may be less detectable than retained intact GBCAs that have higher R1-relaxivity. Finally, T1 signal increases are merely suggestive of Gd retention, and T1 hyperintensity might alternatively reflect various disease-related processes.47⇓–49 While imaging studies can be considered, at best, an indirect second-level marker of Gd deposition, a true picture can only come from direct tissue analysis. In this regard, studies have demonstrated measurable Gd not only in the DN and GP but also in other brain areas and body organs.14,20,28 Most important, Murata et al20 have shown that deposition occurs with both linear and macrocyclic GBCAs and that it is up to 23 times higher in organs such as bone than in the brain.
Despite excellent interreader agreement regarding the reproducibility of SI measurements and despite the absence of significant differences in SI values between gadobenate-exposed and GBCA-naïve control subjects across any of the 4 evaluated brain regions, a significant difference in the GP-thalamus SI ratio was nevertheless still observed in 1 of the 5 blinded assessments. Although this can be explained by higher T1 signal in the thalamus of control subjects, it highlights the potential impact of even minimal differences in SI measurements on study interpretation. This, in turn, points to the importance of multiple readers when performing quantitative evaluations and to the dangers of drawing conclusions based on evaluations performed by just 1 or 2 readers, particularly if such readers are not blinded to information regarding the images under evaluation. Accurate placing of ROIs for quantitative assessment is a highly subjective procedure, which is susceptible to considerable interreader variation.
Our study has some limitations. First, this was a single-center study. Second, because we assessed nonneurologic patients who had not previously undergone MR imaging of the brain, it was not possible to compare unenhanced T1-weighted images after multiple gadobenate administrations with baseline unenhanced images acquired before the first gadobenate administration. However, the lack of significant SI differences between gadobenate-exposed and GBCA-naïve control subjects suggests that no differences would have been seen. Third, we determined DN-pons and GP-thalamus SI ratios despite Gd retention being observed in both the pons and thalamus.14,20 However, the T1 signal changes in these brain areas are much lower than in the DN and GP, and these ratios are commonly calculated parameters.1⇓⇓–4,8⇓⇓⇓⇓–13,16⇓–18 Finally, we did not normalize the SI values of the DN and GP against the SI of the CSF to account for possible intra- and intersequence signal-intensity differences, differences between MR units, and magnetic field inhomogeneity as described by McDonald et al.14 SI normalization might be appropriate for future studies.
Conclusions
Our study of 34 pediatric patients who received between 5 and 15 administrations of 0.05 mmol/kg of body weight gadobenate revealed no differences in T1 signal in the DN, GP, pons, and thalamus relative to measurements in 24 age- and weight-matched control subjects who had never been exposed to any GBCA. Likewise, no meaningful differences were seen in the DN-pons and GP-thalamus SI ratios. If T1 hyperintensity and Gd retention in deep brain nuclei occur in an exposure-dependent fashion, with greater T1 shortening observed following greater prior exposure to GBCAs,1 it would seem prudent to administer the lowest possible dose of a GBCA to achieve diagnostically valid studies, particularly when repeat MR imaging studies are required for follow-up or screening purposes. To this end, a recent intraindividual crossover study in which patients received 2 MR imaging contrast agents in 2 otherwise identical MR imaging examinations has demonstrated similar diagnostic imaging performances for a half-dose (0.05 mmol/kg of body weight) of gadobenate relative to a full dose (0.1 mmol/kg of body weight) of the standard relaxivity macrocyclic GBCA, gadoterate, for morphologic imaging of brain tumors.23 Similarly, a prior study50 demonstrated significant superiority for a three-quarter dose (0.075 mmol/kg of body weight) of gadobenate relative to a full dose (0.1 mmol/kg of body weight) of gadoterate for cranial MR imaging. In both studies, similar or improved imaging performance was achieved with a lower total administered dose of gadolinium when gadobenate was used. We consider half-dose gadobenate safe and effective for diagnosis and routine follow-up of pediatric oncologic patients.
Footnotes
Disclosures: Guenther K. Schneider—UNRELATED: Grants/Grants Pending: Bracco, Siemens, Bayer, Comments: research/clinical studies*; Payment for Lectures Including Service on Speakers Bureaus: Bracco, Siemens. Jonas Stroeder—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Bracco, Comments: allowance for lecture given in June 2014. Giles Roditi—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Bayer, Bracco, Comments: speaker honoraria once for each company for lecturing in educational courses. Cesare Colosimo—UNRELATED: Consultancy: Bracco, Bayer. Arno Buecker—UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Bracco. *Money paid to the institution.
References
- Received January 6, 2017.
- Accepted after revision April 24, 2017.
- © 2017 by American Journal of Neuroradiology