Abstract
BACKGROUND AND PURPOSE: Regional cerebral blood flow has previously been studied in patients with idiopathic normal pressure hydrocephalus with imaging methods that require an intravenous contrast agent or expose the patient to ionizing radiation. The purpose of this study was to assess regional CBF in patients with idiopathic normal pressure hydrocephalus compared with healthy controls using the noninvasive quantitative arterial spin-labeling MR imaging technique. A secondary aim was to compare the correlation between symptom severity and CBF.
MATERIALS AND METHODS: Differences in regional cerebral perfusion between patients with idiopathic normal pressure hydrocephalus and healthy controls were investigated with pseudocontinuous arterial spin-labeling perfusion MR imaging. Twenty-one consecutive patients with idiopathic normal pressure hydrocephalus and 21 age- and sex-matched randomly selected healthy controls from the population registry were prospectively included. The controls did not differ from patients with respect to selected vascular risk factors. Twelve different anatomic ROIs were manually drawn on coregistered FLAIR images. The Holm-Bonferroni correction was applied to statistical analyses.
RESULTS: In patients with idiopathic normal pressure hydrocephalus, perfusion was reduced in the periventricular white matter (P < .001), lentiform nucleus (P < .001), and thalamus (P < .001) compared with controls. Cognitive function in patients correlated with CBF in the periventricular white matter (r = 0.60, P < .01), cerebellum (r = 0.63, P < .01), and pons (r = 0.71, P < .001).
CONCLUSIONS: Using pseudocontinuous arterial spin-labeling, we could confirm findings of a reduced perfusion in the periventricular white matter, basal ganglia, and thalamus in patients with idiopathic normal pressure hydrocephalus previously observed with other imaging techniques.
ABBREVIATIONS:
- ASL
- arterial spin-labeling
- DWMH
- deep white matter hyperintensities
- iNPH
- idiopathic normal pressure hydrocephalus
- MMSE
- Mini-Mental State Examination
- QRAPMASTER
- Quantification of Relaxation Times and Proton density by Multiecho acquisition of a saturation recovery with Turbo spin-echo Readout
Idiopathic normal pressure hydrocephalus (iNPH) is a condition with balance and gait disturbances, cognitive dysfunction, and urinary incontinence.1 The symptoms can be reversed with implantation of a shunt system, with improvement in 50%–80% of patients.2⇓–4
Regional CBF has been assessed in patients with iNPH to find explanations for the pathophysiology and in search of better methods to identify shunt responders. Several studies have shown reduced perfusion in the frontal cortex, periventricular WM, basal ganglia, thalamus, and cerebellum in patients with iNPH compared with controls, but the results have been divergent.5⇓⇓⇓⇓⇓–11 In a recent study, reduced hippocampal CBF was reported as well.9
These previous studies have used different methods to estimate CBF: SPECT, PET, DSC MR imaging, and CT perfusion.8,9,12,13 These methods require administration of a radioactive tracer or a contrast agent, and SPECT, PET, and CT expose the patient to ionizing radiation.
Pseudocontinuous arterial spin-labeling (ASL) is an MR imaging technique with the advantage of allowing quantitative measurements of CBF without injection of any contrast agent.14 The method is completely noninvasive, with a relatively short scan time (∼5 minutes) and can therefore easily be added to a standard MR imaging protocol. To the best of our knowledge, no previous study has investigated differences in CBF between healthy controls and patients with iNPH with an ASL technique.
The purpose of this study was to use ASL to investigate whether regional cerebral perfusion is reduced in patients with iNPH compared with healthy controls and whether the level of clinical symptoms correlates with CBF.
Materials and Methods
Patients and Healthy Controls
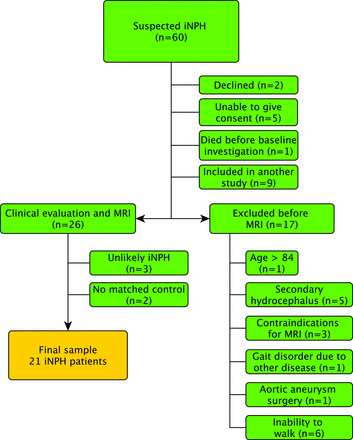
Sixty patients were prospectively and consecutively recruited from the waiting list of patients referred to Uppsala University Hospital for evaluation of suspected iNPH. Nine patients were excluded for participating in an ongoing multicenter study of the CSF tap test. Patients with complete inability to walk were excluded because of the intention to study the relationship between CBF and gait and balance function. Other exclusion criteria were secondary or congenital hydrocephalus, contraindications to MR imaging, older than 84 years of age, severe cognitive dysfunction with an inability to give informed consent, refusal to undergo evaluation or shunt surgery, and gait problems that could be explained by other known diseases. The inclusion process is illustrated in the flow chart (Fig 1). Of the 26 patients examined clinically, 17 were classified as having probable iNPH according to the iNPH guidelines.15 Six patients were classified as having possible iNPH, 1 because of only 1 triad symptom (gait disturbance), 1 due to severe white matter changes, 1 because of a small cyst communicating with 1 of the lateral ventricles, and 3 due to a history of head trauma, probably unrelated to the hydrocephalus. Three patients were considered unlikely to have iNPH, 2 because of symptoms of Parkinson disease and 1 with an aqueductal stenosis. The patients examined in this study have been described in detail in a previous study.16
Flow chart describing the inclusion process of patients.
Controls were randomly recruited from the county of Uppsala using the Swedish population registry and were matched with patients with respect to age (±2 years) and sex. Exclusion criteria were any known neurologic disease, stroke, diabetes mellitus, history of myocardial infarction that required acute treatment or resulted in persistent electrocardiogram changes, dependence on walking aids, or any terminal disease. Antihypertensive medication, aspirin, and common pain medications were allowed. Information was sent to 105 potential elderly controls. Forty-nine did not reply; 31 responded that they did not want to participate or could not participate according to the exclusion criteria. The remaining 25 accepted participation and were examined clinically by a neurologist. MR imaging examination was not possible in 2 of the 25 controls because the MR imaging scanner was upgraded before those 2 controls were examined, and another 2 controls did not match any patient. Thus, a total of 21 matched pairs of patients (15 with probable iNPH and 6 with possible iNPH) and controls were available and included in the statistical analysis. The study was approved by the local ethics committee in Uppsala, Sweden.
Clinical Examination
Neurologists, especially trained physiotherapists, and occupational therapists performed the clinical examinations. Besides a standard neurologic examination, the tests included the modified Rankin Scale, Mini-Mental State Examination (MMSE), incontinence scale,17 Romberg test, and time and number of steps required to walk 10 m at a maximum pace. Tests of motor function were performed twice, and the mean result was used in the statistical analyses.
Imaging
Details of the imaging method, scanner protocol, and postprocessing have been described previously.16 The first MR imaging was performed between 8:00 am and 10:00 am. The second MR imaging was performed 60 minutes after the first scan to assess the repeatability of the method. The patients left the scanner after the first investigation. Between the investigations, the patients rested in the supine position in a quiet, isolated room next to the scanner. The patients were not allowed to use caffeine on the day of the MR imaging, and all transportation was performed with the patient in bed or in a wheelchair to minimize variations in CBF. Healthy controls were only examined once with MR imaging.
Scanner and Imaging Protocol
Imaging was performed with a 3T MR imaging scanner (Achieva; Philips Healthcare, Best, the Netherlands) with a 32-channel head coil. A 3D T1-weighted gradient-echo and a T2-weighted FLAIR sequence were included in the protocol. Perfusion MR imaging was performed with a background-suppressed pseudocontinuous ASL sequence, with a single-shot echo planar imaging readout, with the following protocol: FOV = 264 × 264 mm2, in-plane resolution = 2.75 × 2.75 mm2, section thickness = 5 mm, number of sections = 20, TR = 4100 ms, TE = 14.5 ms, label duration = 1650 ms, postlabeling delay = 1600 ms, and 30 repetitions. To allow quantitative CBF values, we obtained a fast reference scan.
Synthetic MR imaging data were acquired with the multisection, multiecho, and multisaturation delay sequence Quantification of Relaxation Times and Proton density by Multiecho acquisition of a saturation recovery with Turbo spin-echo Readout (QRAPMASTER).18 Because of technical problems, synthetic MR imaging data were not acquired in 2 patients and in 1 control.
Perfusion MR Imaging Postprocessing
For perfusion quantification, the compartment model proposed by Alsop and Detre was used.19 The magnetization difference (perfusion-weighted signal) is given by
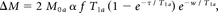 where M0a is the arterial blood magnetization, α is the labeling efficiency, f is the perfusion, T1a is the T1 of arterial blood, τ is the labeling duration, and w is the postlabeling delay. Labeling efficiency was set to 70%, T1a was set to 1664 ms, and M0a was estimated with the signal intensity of CSF (see Alsop and Detre19 for more detail).
where M0a is the arterial blood magnetization, α is the labeling efficiency, f is the perfusion, T1a is the T1 of arterial blood, τ is the labeling duration, and w is the postlabeling delay. Labeling efficiency was set to 70%, T1a was set to 1664 ms, and M0a was estimated with the signal intensity of CSF (see Alsop and Detre19 for more detail).
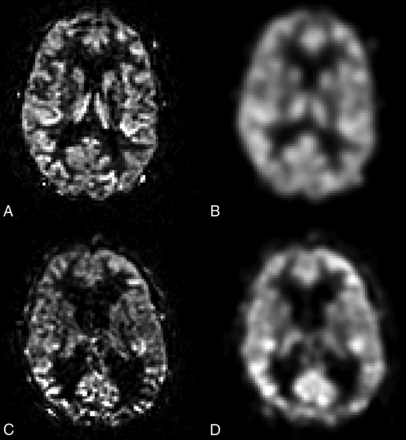
As an anatomic reference, the FLAIR images were coregistered to the perfusion maps by using a 12-degree affine transform (elastix; http://elastix.isi.uu.nl/). To minimize the effect of nonideal image coregistration and to reduce the intrasubject variation caused by varying delineation of regions, we spatially smoothed the CBF maps (Gaussian kernel with 4-mm full width at half maximum, Fig 2).
Example of ASL perfusion maps from a healthy control without (A) and with (B) smoothing, as well as from a patient with iNPH without (C) and with (D) smoothing.
ROIs and Morphologic Evaluations
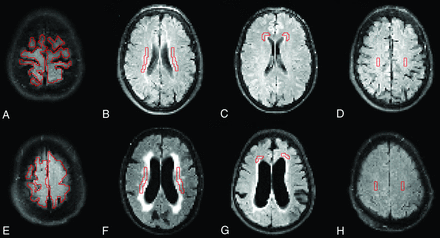
Twelve different ROIs were drawn manually on transverse T2 FLAIR images using the in-house software Eval Gui (developed by Markus Nilsson, Lund University, Lund, Sweden). We covered the following regions: cerebellum, pons, high-convexity cortex, medial frontal cortex, lentiform nucleus, medial temporal lobe, supplementary motor area, thalamus, frontal WM, lateral WM, superior WM, and periventricular WM. The number of sections and voxels for all ROIs is listed in Table 1. Four different ROIs are illustrated in Fig 3, and all ROIs in both patients and controls are illustrated in the On-line Appendix. All sections of all scans were visually inspected, and care was taken not to include artifacts or large vessels in the ROIs. The ROIs were drawn by the first author (J.V., 4 years of experience) and reviewed by the last author (E.-M.L., >30 years of experience). When ROIs were drawn, the investigator was blinded to the patients' clinical data. CBF values were calculated for every voxel, and a mean CBF was provided in each ROI.
Cerebral blood flow values in patients and controlsa
FLAIR images that were coregistered to the ASL images and used to draw ROIs. A–D, Healthy controls. E–H, Patients with iNPH. A and E, High-convexity cortex. B and F, Lateral periventricular WM. C and G, Frontal WM. D and H, Superior WM. All ROIs above were drawn in 2 adjacent sections, 1 of which is shown here.
For descriptive purposes, we assessed the following morphologic MR imaging features: the Evans index,20 the callosal angle,21 and deep white matter hyperintensities (DWMH) according to the visual grading scale of Fazekas et al.22 Disproportionately enlarged subarachnoid space hydrocephalus was defined as a combination of enlarged ventricles (Evans index of >0.3), narrow sulci at the high convexity or parafalcine and dilated Sylvian fissures (Table 2).3 The measurements of morphologic features were performed by the last author (E.-M.L.). She was blinded to CBF data.
Demographics and background data in patients and controlsa
Volumetric Measurement
Measurements of CSF volumes were performed by using the QRAPMASTER sequence,18 which provides quantification of longitudinal relaxation time (T1), transverse relaxation time (T2), and proton density. The software SyMRI Brain Studio (SyntheticMR, Linköping, Sweden) uses combinations of T1, T2, and proton density to estimate voxelwise partial volumes of GM, WM, and CSF, which allow volumetric estimation of these tissues. The volume of both lateral ventricles was calculated semiautomatically in both patients and controls after manual delineation on each section that included the lateral ventricles.
Statistical Analysis
The mean CBF in each ROI from the 2 scans of the patients was used in the statistical analyses. Each patient was paired with an age- and sex-matched control. A paired-samples t test was used for the difference in CBF between patients and controls. Differences in descriptive data and comorbidity were analyzed with the Wilcoxon signed rank test or the McNemar test. Difference in age was tested with the Mann-Whitney U test. Correlations were assessed with the Spearman correlation. An intraclass correlation coefficient (2-way mixed, single measures) was used to calculate the repeatability of CBF between the 2 investigations in patients. For the analysis of CBF in patients and controls in the 12 ROIs, the Holm-Bonferroni correction for multiple comparisons was applied to reduce type I errors. Holm-Bonferroni correction works by sorting the N P values from the multiple tests and testing the smallest P value against α/N, the second smallest against α/(N-1), and so on. As soon as a null hypotheses is not rejected, all subsequent null hypotheses are also deemed not rejected, resulting in an effective significance threshold. With α = .05, the effective significance threshold for ROI analysis in patients and controls was P < .006. Correlations were tested among all 12 ROIs, and the 4 clinical tests as well as the 4 morphologic imaging features. Only correlations with r > 0.60 were considered strong and included in the results section. All analyses were performed with SPSS Statistics for Macintosh, Version 22.0 (IBM, Armonk, New York).
Results
Background Data
The radiologic morphologic measurements and the results of gait and balance tests, urgency, and MMSE differed markedly between patients and healthy controls (Table 2). However, there were no significant differences between patients and controls concerning age, the radiologic finding DWMH, and the use of antihypertensive drugs, acetylsalicylic acid, antidiabetic drugs, or statins, and there were no differences in the history of stroke, traumatic brain injury, meningitis, and previous or present smoking.
Cerebral Blood Flow
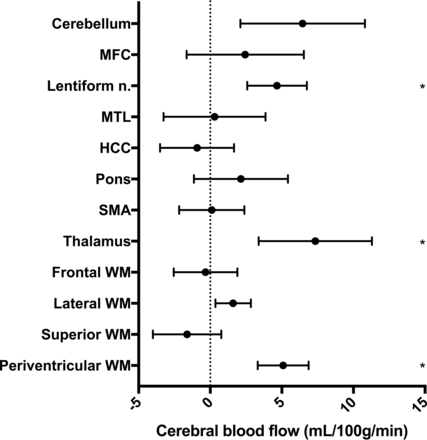
The CBF in all patients and controls is presented in Table 1. In patients, the CBF was significantly lower in the periventricular WM, lentiform nucleus, and thalamus after Holm-Bonferroni correction (Fig 4). In 3 patients with iNPH, the ASL maps contained vascular artifacts and apparent hypoperfusion in the brain parenchyma. If these 3 patients were excluded from statistical analyses, CBF was still significantly lower in patients in the periventricular WM, lentiform nucleus, and thalamus. There was no difference in age or symptom severity among the 3 patients with vascular artifacts compared with the other patients.
Mean difference with 95% CI between patients with iNPH and healthy controls. MFC indicates the medial frontal cortex; HCC, high-convexity cortex; MTL, medial temporal lobe; SMA, supplementary motor area. The asterisk indicates a significant difference after Holm-Bonferroni correction.
Morphologic Features and CBF
In controls, there were negative correlations between the Evans index and perfusion in frontal WM (r = −0.75, P < .001), lateral WM (r = −0.73, P < .001), and periventricular WM (r = −0.66, P < .01). In controls, there were also negative correlations between quantified lateral ventricular volume with Synthetic MR imaging and perfusion in the frontal WM (r = −0.72, P < .001), lateral WM (r = −0.67, P < .01), periventricular WM (r = −0.65, P < .01), and medial frontal cortex (r = −0.61, P < .01). There were no correlations between ventricular volume and CBF in patients.
Clinical Symptoms and CBF
In patients, the MMSE score correlated significantly with CBF in the pons (r = 0.71, P < .001), cerebellum (r = 0.63, P < .01), and periventricular WM (r = 0.60, P < .01), but not in controls. Neither in patients nor in controls did gait, balance, or urgency incontinence correlate with CBF values. See On-line Tables 1 and 2 for all correlation coefficients in patients.
Repeatability
Repeatability for the CBF values obtained in the 2 investigations in patients is presented in Table 1 as intraclass correlation coefficients. The intraclass correlation coefficients were in the range of 0.72–0.92.
Volumetric Measurement
The median lateral ventricular volume in patients was significantly larger than in controls, 130 mL (interquartile range = 111–136 mL) and 31 mL (interquartile range, 24–54 mL), respectively.
Discussion
In this study, pseudocontinuous ASL, a noninvasive perfusion method without ionizing radiation or contrast agent, was used to compare cerebral perfusion between patients with iNPH and healthy controls. In agreement with previous studies using anatomic ROIs in patients with iNPH, CBF was reduced in the periventricular WM, basal ganglia, and thalamus.5,9 There was a trend indicating that CBF was also reduced in the cerebellum in patients with iNPH, but the difference was not significant after Holm-Bonferroni correction. In patients, MMSE correlated significantly with CBF in the pons, cerebellum, and periventricular WM. The repeatability of the CBF measurements was acceptable to high, which strengthens our findings.
In the present study, reduced CBF in patients with iNPH was found in the basal ganglia, thalamus, and periventricular WM, whereas previous studies also reported hypoperfusion in the frontal cortex, temporal lobes, and cerebellum.5,9,13 The more conservative correction for multiple analyses performed in our study could be one explanation for this difference, but another could be differences in the selection of healthy controls as well as the difference in imaging methods.
The mechanism of the reduced regional CBF in iNPH is likely multifactorial. One hypothesis is that transependymal passage of CSF into the parenchyma leads to reversal of interstitial fluid flow, initiating local CSF edema in the periventricular WM. The accumulation of interstitial fluid may cause local compression of small vessels and, more important, reduce elimination of vasoactive metabolites. The region mainly affected by hypoperfusion, according to this hypothesis, should be the periventricular WM, which is in line with our results and has also been reported by others.6,9 The reduced CBF in the thalamus and lentiform nucleus may be an indirect result of periventricular WM edema, by affecting the penetrating arteries that supply the thalamus and lentiform nucleus.
The supplementary motor area, basal ganglia, thalamus, mesencephalon, or the white matter tracts connecting these regions have been suggested as the most important anatomic structures behind gait and balance disturbances in patients with iNPH.23,24 There were no correlations between gait function and CBF in the present study. These findings indicate that the etiology of the gait disturbance in iNPH is complex and might be caused by factors other than reduced CBF.
However, there were correlations in the present study between impaired cognitive function measured with MMSE and reduced CBF in the pons, cerebellum, and periventricular WM. Perfusion in the brain stem has not been well-studied in iNPH, but Tullberg et al25 reported a relative CBF increase in the mesencephalon in patients with improved wakefulness after shunt surgery. Disturbances in cognitive function, especially in attention and executive ability, have been reported after insults to the pons,26 and such symptoms are often seen in iNPH as well.27 The connection between impaired cognitive function and reduced CBF in the cerebellum is harder to explain; however, there are reports that the cerebellum is involved in cognitive function.28,29
Hypertension and vascular risk factors30 and MR imaging evidence of vascular disease such as DWMH are overrepresented in patients with iNPH.31 These vascular risk factors are associated with reduced CBF32; therefore, differences in CBF could be overestimated if controls are a highly selected sample of healthy elderly. In the present study, the controls were randomly selected from the population, with a limited number of exclusion criteria. There was no significant difference in the burden of DWMH or the use of acetylsalicylic acid or antihypertensive drugs between patients and controls, which strengthens our findings.
In controls, there were correlations between the size of the lateral ventricles and reduced CBF in WM regions. A potential reason may be asymptomatic small-vessel disease with reduction of WM volume and secondary dilation of the lateral ventricles, indicating that even moderately enlarged ventricles may not be considered a normal aging phenomenon.
The pseudocontinuous ASL technique has some advantages compared with other perfusion methods. The repeatability in healthy controls and in patients with Alzheimer disease is high, and CBF values have been validated against PET.33 Like PET, perfusion values can be quantified. Therefore, no reference regions are needed to calculate relative perfusion values. In SPECT and DSC perfusion, relative values are used, with the risk that pathologic perfusion in the reference regions may affect the relative perfusion values. In a recent study, CT perfusion was evaluated in iNPH and may be a promising alternative because it is available in many sites and is not as sensitive to artifacts from the shunt valve after shunt implantation as with MR imaging.8
In the iNPH guidelines from 2005, the SPECT-acetazolamide challenge is the only perfusion method mentioned as a supportive diagnostic technique.15 A SPECT-acetazolamide challenge requires a separate imaging investigation, is time-consuming, and exposes the patient to ionizing radiation from the injected tracer. The ASL sequence is rapid and noninvasive; these features make it ideal for research studies in patients with iNPH, but the diagnostic value of the method is uncertain. Although CBF differences were found between patients and controls on a group level, the effect may not be large enough in individual cases to allow the use of ASL as a supportive diagnostic technique in clinical routine.
The strength of this study was that prospectively and consecutively included patients were compared with age- and sex-matched controls who were randomly selected from the population.
Some limitations need to be considered. Three patients had a high intravascular signal with low signal intensity in the brain parenchyma, probably due to a longer arterial transit time than postlabeling delay.34 Statistical analysis with and without these 3 patients did not show any significant differences; therefore, we chose not to exclude them. Individually optimized postlabeling delays would be preferable in elderly patients with pathologic CBF.
The CBF values in both our patients and controls were low compared with those reported in PET studies,5,35 but they were in line with other ASL studies in patients with iNPH.36 Also, it has been reported that quantified perfusion values are underestimated in elderly individuals with ASL.37 Our main results were probably not affected by this limitation because we compared patients with age-matched controls.
White matter perfusion estimation with ASL is challenging, due to a longer arterial transit time and lower blood volume compared with gray matter. Thus, the WM perfusion signal had a low signal-to-noise ratio, and the corresponding results should be treated with some caution. On the other hand, 2 recent studies suggest that though pixel-wise analysis is challenging, ROI-based analysis of WM perfusion is feasible in single subjects.38,39 This finding increases the credibility of our group-level results based on ROI analysis in 21 patients and 21 controls.
Spatial smoothing was applied to reduce the effects of nonideal coregistration but can potentially introduce partial volume effects on CBF measurements. This might have influenced the results of small ROIs with contamination of data from surrounding tissue.
The MMSE cognitive test used in this study may underestimate subcortical deficits that are very relevant in iNPH. More sophisticated neuropsychological tests could have revealed more information about the relationship between cognition and CBF in both patients and controls.40
Conclusions
Pseudocontinuous ASL was used to compare perfusion of patients with iNPH versus healthy controls. In patients, perfusion values were reduced in the periventricular WM, basal ganglia, and thalamus, and there was a correlation between cognitive dysfunction and reduced CBF. Because ASL is a noninvasive quantitative perfusion method without ionizing radiation or contrast agent, it is a suitable perfusion method for research studies in patients with iNPH.
Acknowledgments
The authors thank Markus Nilsson for the software Eval Gui, Markus Fahlström for technical assistance, and Emma Jansson for assistance with examinations of healthy controls. The authors also thank our normal pressure hydrocephalus team and the MR imaging staff at Uppsala University Hospital, especially Britt-Mari Bolinder. Finally, the authors thank Selanders Stiftelse for its support.
Footnotes
Disclosures: Johan Virhammar—RELATED: Grant: Selanders Foundations, Comments: Swedish independent foundation*; UNRELATED: Payment for Lectures Including Service on Speakers Bureaus: Medtronic, Comments: I have received 1 educational lecture honoraria from Medtronic for 1 lecture. André Ahlgren—UNRELATED: Grant: Swedish Research Council.* *Money paid to the institution.
Johan Virhammar was supported by the independent Swedish foundation Erik, Karin och Gösta Selanders Stiftelse; Katarina Laurell received research grants from the Region Jämtland Härjedalen.
References
- Received March 16, 2017.
- Accepted after revision June 12, 2017.
- © 2017 by American Journal of Neuroradiology