Abstract
BACKGROUND AND PURPOSE: Spinal dural arteriovenous fistulas are commonly missed on imaging or misdiagnosed as inflammatory or neoplastic processes. We reviewed a consecutive series of spinal dural arteriovenous fistulas referred to our institution that were missed or misdiagnosed on initial imaging and studied the clinical consequences of missing or misdiagnosing the lesion.
MATERIALS AND METHODS: We reviewed spinal dural arteriovenous fistulas diagnosed at our institution between January 1, 2000, and November 1, 2014. A lesion was defined as “misdiagnosed” if initial MR imaging or CT myelography demonstrated characteristic imaging features of spinal dural arteriovenous fistula but the patient was clinically or radiologically misdiagnosed. Outcomes included length of delay of diagnosis, increased disability (increase in mRS or Aminoff motor disability of ≥1 point) between initial imaging evaluation and diagnosis date, and posttreatment disability.
RESULTS: Fifty-three consecutive spinal dural arteriovenous fistulas that were initially misdiagnosed despite having characteristic imaging findings on MR imaging or CT myelography were included in our study. Eight patients (18.9%) underwent spinal angiography before referral, which was interpreted as having negative findings but was either incomplete (6 cases) or retrospectively demonstrated the spinal dural arteriovenous fistulas (2 cases). The median time of delayed diagnosis was 6 months (interquartile range, 2–14 months). Fifty-one patients (96.2%) had increased disability between the initial study, which demonstrated features of a spinal dural arteriovenous fistula, and diagnosis. Thirty-two patients (60.4%) developed a new requirement for a walker or wheelchair. Following treatment, 21 patients (41.2%) had an improvement of 1 point on the mRS or Aminoff motor disability scale.
CONCLUSIONS: Delayed diagnosis of spinal dural arteriovenous fistula with characteristic imaging features results in high rates of additional disability that are often irreversible despite surgical or endovascular treatment of the fistula.
ABBREVIATION:
- SDAVF
- spinal dural arteriovenous fistula
Spinal dural arteriovenous fistulas (SDAVFs) are spinal vascular lesions that classically present with vague symptoms such as leg dysesthesias and exertional leg weakness but slowly progress to severe myelopathy with paraplegia and sphincter dysfunction.1 Because of the nonspecific nature of their presenting symptomatology and insidious onset, SDAVFs often go clinically undiagnosed or are misdiagnosed as peripheral neuropathy, spinal stenosis, multiple sclerosis, transverse myelitis, or radiculopathies.2
Despite the vague clinical symptoms associated with the initial presentation of SDAVFs, they typically demonstrate a characteristic imaging appearance. On MR imaging, SDAVFs are characterized by spinal cord enlargement in the lower thoracic region and conus, with T2 hyperintensity across multiple segments and serpiginous, enlarged intradural vessels along the dorsal and ventral aspect of the cord. Gadolinium-enhanced spine MR imaging is often helpful in highlighting the dilated intradural veins and can even demonstrate cord enhancement but is not indispensable for radiologic diagnosis.3 Given the characteristic imaging features of these lesions, they represent a prime opportunity for the radiologist to make an important and often clinically unsuspected diagnosis.
Despite their classic imaging appearance, SDAVFs can remain undiagnosed on imaging or can be misdiagnosed. In a number of cases in the literature, high T2 signal in the conus or prominent vascular flow voids in the intradural space are missed on initial imaging, only to be picked up at follow-up imaging after progression of the patient's symptoms. In addition, cases exist in which the cord signal was hyperintense on T2-weighted images and the patient was diagnosed with and treated for transverse myelitis or neuromyelitis optica, despite the presence of flow voids.2,4,5
In this study, we examined a consecutive series of patients presenting with imaging findings of SDAVFs that were missed or misdiagnosed. We studied the clinical consequences of delayed diagnosis, such as progression of disability, use of additional imaging, number of months until diagnosis, and improvement of clinical symptoms following treatment.
Materials and Methods
Patient Population
Following institutional review board approval, we reviewed a consecutive series of angiographically confirmed SDAVFs diagnosed and/or treated at our institution from January 1, 2000, to November 1, 2014. Patients with clinically or radiologically misdiagnosed SDAVFs had to meet at least 1 of the following criteria as determined by 2 reviewers: 1) Initial MR imaging or CT myelogram of the spine demonstrated characteristic imaging features of an SDAVF, but these findings were not noted in the radiology report; 2) initial MR imaging or CT myelogram of the spine demonstrated characteristic imaging features of an SDAVF, no radiology report was available, and the patient was treated initially for a disease other than an SDAVF without conventional angiography, spinal MRA, or CTA being performed; or 3) an SDAVF was suspected on the basis of imaging, and the patient had a spinal angiography that was incomplete (ie, not all vessels were injected) or showed an SDAVF that was not appreciated, leading to the incorrect interpretation of negative findings.
Our institution is a large tertiary referral center, so most patients received extensive evaluations at other centers before arriving at our institution. Because radiology reports did not always accompany images from outside centers (only 5 cases with outside radiology reports were available), the clinical management of the patient was most often used to indicate whether the SDAVF was missed or misdiagnosed. The definition of characteristic imaging features of SDAVF was high T2 cord signal and/or serpiginous vascular intradural flow voids with or without intramedullary enhancement on MR imaging or a serpiginous blood vessel coursing in the spinal canal on CT myelography. Vascular intradural flow voids were distinguished from CSF pulsations because these were serpiginous flow voids taking the expected shape/morphology of a blood vessel.
Imaging Evaluation
Imaging examinations were simultaneously reviewed by a staff neuroradiologist with 10 years' experience and a senior diagnostic radiology resident. The readers were not blinded to the patient's diagnosis of an SDAVF. MR imaging examinations were reviewed for the following imaging findings: increased T2 signal in the conus, number of levels of high T2 signal, presence of flow voids, and presence of cord enhancement on postgadolinium images. “Serpiginous flow voids” were defined as serpiginous areas of signal loss on T2-weighted imaging. This definition was chosen because such flow voids are not usually seen on T2-weighted images around the lower spinal cord/conus medullaris. “Intramedullary” enhancement was defined as enhancement of the conus or spinal cord itself rather than surface enhancement of pial vessels, which can be seen in some as normal findings. We compiled imaging findings and scored them by using the recently validated 4-point arteriovenous fistula score, which assigns 1 point to the following clinical/imaging characteristics: 1) 50 years of age or older, 2) T2 hyperintensity extending to at least 5 levels, 3) flow voids, and 4) a subcervical lesion.6 A score of ≥3 has been found to have a sensitivity of 85% and specificity of 97% in determining the presence of an SDAVF.6 CT myelograms were evaluated for the presence of a serpiginous vessel coursing in the intradural space. Conventional spinal angiograms were evaluated for the presence of an SDAVF and angiogram completeness. An angiogram was defined as “incomplete” if the feeding artery seen on a later angiogram was not selectively injected. We also evaluated posttreatment angiograms and spinal MR angiograms for resolution of the SDAVF.
Clinical Evaluation
The clinical evaluation was performed by a vascular neurology fellow (D.M.N.) through a retrospective chart review of clinical notes and neurologic examinations. We collected the following clinical data: time of delay in diagnosis; modified Rankin Scale score on presentation, diagnosis, and 90 days posttreatment; Aminoff score of motor disability at presentation, diagnosis, and 90 days posttreatment; worsening of symptoms (defined as an increase of at least 1 point on the mRS or Aminoff score); interval use of a walker following initial imaging; interval use of a wheelchair following initial imaging; and interval development of sensory symptoms (including dysesthesias and paresthesias) or bowel and bladder symptoms (including neurogenic bladder and incontinence).
Additional Procedures and Imaging
In addition to documenting the type of treatment for the SDAVF, we also collected data on additional imaging and interventions performed before the diagnosis of SDAVF. Data collected on interventions included the use of systemic steroids, IV immunoglobulin, and plasmapheresis between the initial imaging study that demonstrated evidence of the SDAVF and the time the fistula was actually recognized. We also tabulated the number of additional imaging studies that were performed before arriving at the diagnosis of SDAVF.
Statistical Analysis
Because this was a descriptive study, no formal statistical comparisons were performed. We report descriptive statistics including mean, median, and proportions. All analyses were performed with the JMP statistical software package, Version 10.0 (SAS Institute, Cary, North Carolina).
Results
Patient Population
One hundred patients were diagnosed with SDAVFs at our institution during this time. Of these, 53 (40.8%) met our inclusion criteria. The mean age of these patients was 65.0 ± 10.8 years, and 48 patients (90.6%) were older than 50 years of age. Forty-three patients were men (81.1%), and 10 patients were women (18.9%). The mean number of months between the time when the first imaging study demonstrating findings of SDAVF was performed and the time of diagnosis was 9.2 ± 11.1 months (median, 6 months). The 3 most common working diagnoses for patients who had imaging evidence of SDAVF were spinal stenosis (13 patients, 24.5%), myelopathy not otherwise specified (10 patients, 18.9%), and transverse myelitis (9 patients, 17.0%).
Systemic steroid administration (oral or IV) was the most common medical intervention between the initial imaging study demonstrating the SDAVF and the time of diagnosis (18 patients, 34.0%). Six patients (11.3%) had laminectomies during this interval, 1 patient had a spinal cord biopsy, and 4 patients (7.6%) had plasma exchange. The median number of additional imaging studies performed before diagnosis was 3. Eleven patients (20.8%) had ≥5 additional imaging studies. These data are summarized in Table 1.
Patient characteristics and additional procedures
Imaging Findings on Arteriovenous Fistula Score
Forty-eight patients (90.6%) had evidence of SDAVFs on MR imaging on initial imaging evaluation, and 5 patients (9.4%) had imaging evidence of SDAVF on CT myelography. Forty-four patients (91.7%) had high T2 signal in the conus on MR imaging, and 46 patients (95.8%) had increased T2 signal in the cord. Thirty-seven patients (77.1%) had at least 5 levels of high T2 cord signal intensity. In 43 patients (89.6%), the high T2 cord signal extended ≥3 levels. Fifty-one patients (96.2%) had flow voids in the intradural space on MR imaging or a dilated serpiginous vessel in the intradural space on CT myelography. Thirty-eight patients (79.2%) had spinal cord enhancement. Forty-four patients (91.7%) had both high cord T2 signal and flow voids. In total, 51 patients (96.1%) had an arteriovenous fistula score of ≥3, indicating a high probability of a spinal dural arteriovenous fistula.
Two patients had high T2 signal but no flow voids. In 1 case, the spinal MR imaging had high T2 cord signal extending 6 levels and intramedullary enhancement. The patient was 80 years of age. This patient had an arteriovenous fistula score of 3. The patient was misdiagnosed as having an ischemic myelopathy. The second patient was a 49-year-old man with high T2 conus signal that was interpreted as myelomalacia and cord atrophy due to spinal stenosis. The patient underwent a laminectomy, but his symptoms progressed. Six months following the initial MR imaging, the patient underwent a spinal MRA due to suspicions that his symptoms were due to an SDAVF on the basis of the gradual progression of his symptoms. This patient had an arteriovenous fistula score of 1.
Eight patients (18.9%) underwent spinal angiographies that were interpreted as having negative findings. In all 8 cases, patients were treated for diagnoses other than SDAVF following angiography. In 6 cases (11.3%), the feeding artery was not selectively injected and no SDAVF was visualized. Among these 6 cases, in 2 cases the feeding artery was a hypogastric/internal iliac artery, and in 4 cases, the feeding artery was a radiculomeningeal artery from the lumbar or thoracic levels. In 2 cases, the fistula was present because the angiogram of the culprit artery was selectively injected; however, the angiogram was interpreted as negative. One additional patient had a 5-year delay in the diagnosis of an SDAVF despite imaging findings of high T2 cord signal and flow voids. Imaging findings are summarized in Table 2. Case examples are shown in Figs 1 and 2.
Imaging characteristics
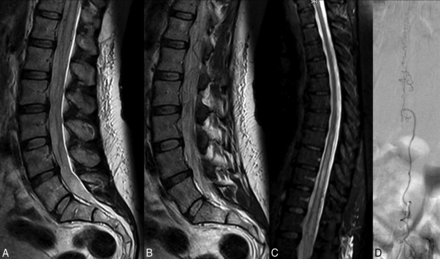
A 57-year-old woman with a 3-month history of bilateral lower extremity tingling and progressive lower extremity weakness. A and B, T2-weighted lumbar spine MR images demonstrate high T2 signal in the conus with multiple flow voids in the intradural space. C, T2-weighted MR image of the thoracic spine demonstrates high T2 signal in the lower thoracic cord to the conus. The patient was diagnosed with neuromyelitis optica and received no spinal-vasculature imaging before referral to our institution. Two rounds of IV methylprednisolone (Solu-Medrol) therapy resulted in worsening of symptoms, and rituximab therapy was of no benefit. D, Spinal angiography demonstrates the spinal dural AVF with an arterial feeder from the L3 radiculomeningeal artery.
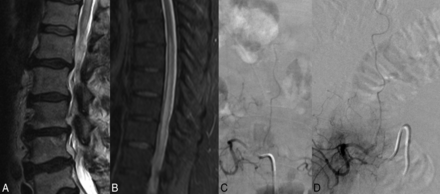
A 68-year-old man with a 3-month history of saddle anesthesia, constipation, difficulty voiding, and numbness in the lower extremities. T2-weighted images of the lumbar and thoracic spine demonstrate high T2 signal in the lower thoracic cord and conus (A and B). Due to clinical suspicion of SDAVF, an angiogram was obtained before referral to our center. C, The angiogram clearly demonstrates the fistula arising from the L2 radiculomeningeal artery; however, it was interpreted as a negative finding. Before the diagnosis was made, the patient underwent an extensive imaging and clinical evaluation, including a panel negative for paraneoplastic syndrome, PET/CT, and lumbar puncture. Two rounds of IV Solu-Medrol therapy resulted in worsening of symptoms. The patient also underwent a T10–T11 laminectomy and 2 spinal cord biopsies. D, Repeat spinal angiography re-demonstrates the fistula.
Clinical Progression and Posttreatment Outcomes
At the time of initial imaging evaluation, 51 patients (96.3%) had some motor deficit, 20 patients (37.7%) had sensory symptoms including pain, and 13 patients (24.5%) had bowel or bladder symptoms. At the time of diagnosis, 51 patients (96.2%) had worsening of disability as measured by the mRS and 51 patients (96.2%) had worsening of motor disability as measured by the Aminoff score of motor disability. Forty of 42 patients (95.2%) with an mRS of 0–1 at baseline had worsening of symptoms at the time of diagnosis, and 25 of 35 patients with an Aminoff score of motor disability of 0–1 at baseline had worsening of symptoms at the time of diagnosis (71.4%). Seventeen patients (32.1%) required a wheelchair, 15 patients (28.3%) required a walker, and 15 patients (28.3%) required a cane.
Forty-one patients (77.4%) underwent surgical ligation for initial treatment, and 11 patients (20.8%) underwent endovascular embolization. Forty-eight patients had postoperative MRA or spinal angiography, and resolution of the SDAVF on imaging was seen in 46 patients (95.8%). Ninety days following treatment, 29 patients (56.8%) demonstrated no improvement in disability as measured by the mRS and 21 patients (41.2%) had an improvement of at least 1 point on the mRS scale. One patient died due to complications from metastatic gastric adenocarcinoma (2.0%). On the Aminoff score of motor disability, 47 (94%) patients were either stable (31 patients, 62.%) or improved (16 patients, 32%) after treatment, while 3 patients (6.0%) demonstrated worsening of their motor disabilities. At 90 days posttreatment, 12 patients (24.0%) required a wheelchair, 11 patients (22.0%) required a walker, and 14 patients (28.0%) required a cane. These data are summarized in Table 3.
Clinical outcomes
Discussion
Our study of 53 patients with a delayed diagnosis of SDAVF demonstrated a high rate of additional morbidity and disability from the time of imaging evidence of SDAVF to the time of diagnosis. Notably, all patients in our cohort could ambulate independently at the time of the initial imaging study demonstrating the SDAVF, but more than half (32 patients) had become wheelchair-bound or required a walker by the time the SDAVF was recognized. Furthermore, this progression of disability was usually not reversible with treatment of the SDAVF: Only one-third of patients becoming wheelchair-bound by the time of SDAVF diagnosis were able to ambulate with a walker 90 days after treatment. The morbidity associated with delayed diagnosis of SDAVF was likely preventable in most patients. Approximately 90% of patients with a delayed diagnosis had both serpiginous flow voids and high T2 cord signal on the initial imaging evaluation, which are the classic imaging characteristics of SDAVFs, and 96% of patients had abnormal intradural spinal vessels. These findings highlight the importance of a timely diagnosis of SDAVFs and the role of the radiologist in raising clinical suspicion for these lesions.
In general, SDAVFs are associated with a poor natural history. It has been proposed that SDAVFs result from loss of normal physiologic control of the glomerulus of Manelfe, a structure located between 2 layers of the dura mater, composed of ≥2 arterioles converging with a vascular ball (glomerulus) and being drained by a single intradural vein. However, the means by which the glomeruli of Manelfe lose their ability to be physiologically controlled is still unknown. Following formation of the fistula between the radiculomeningeal artery and radicular vein, venous congestion along the longitudinal venous network draining the spinal cord can occur. Congestion is generally most marked in the conus due to its dependent location, resulting in the classic sensorimotor deficits and bowel and bladder symptoms.7 Progression of the lesion results in increased venous congestion and, in turn, chronic hypoxia and progressive myelopathy with worsening of symptoms.8 The onset of symptoms is often insidious and can take place years after the fistula develops.9 In general, when left untreated, symptomatic SDAVFs progress to severe irreversible myelopathy with paraparesis and sphincter dysfunction. However, the natural history of asymptomatic, incidentally discovered SDAVFs is unknown.10
So many of these lesions are diagnosed late or misdiagnosed because of the slow and insidious onset of symptoms. SDAVFs have been reported misdiagnosed and even treated as peripheral neuropathy, radiculopathies, multiple sclerosis, intramedullary tumors, neuromyelitis optica, and transverse myelitis.11,12 Furthermore, because of the demographic characteristics of patients affected by these lesions (typically older men), they are often misdiagnosed as central spinal canal stenosis secondary to degenerative changes (ie, spinal stenosis), as occurred in approximately 20% of patients in our study. With or without initial misdiagnosis, diagnostic delays are common and can be quite long. In fact, the estimated time from clinical symptom onset to diagnosis ranges from 11 to 27 months, depending on the series.13,14
Because of the protean clinical symptoms of SDAVFs in early stages, imaging plays a central role in the diagnosis of these lesions. As mentioned previously, SDAVFs have a very characteristic imaging appearance, including high T2 conus signal, high contiguous T2 signal in the spinal cord, enhancement, and intradural vascular flow voids.3,15 In our series for example, approximately 80% of patients had evidence of high T2 cord signal and flow voids in their initial imaging study. High conus signal was present in 83% of patients, which is important because many patients undergo lumbar spine imaging as the initial evaluation for their symptoms. High conus or cord signal is not a specific finding for SDAVF; however, when detected, the radiologist should consider the possibility of an SDAVF in the correct clinical setting.
In a retrospective study of 78 patients with unexplained myelopathy, Strom et al16 found that nearly 30% of patients had an SDAVF on angiography. This finding led to the conclusion that spinal angiography should be considered in patients with unexplained myelopathy to allow prompt diagnosis of an SDAVF. Cao et al6 proposed a 4-point score to identify SDAVFs, suggesting that patients who meet at least 3 of the following 4 criteria should be referred for spinal angiography: 50 years of age or older, length of intramedullary lesion ≥5 segments, perimedullary dilated vessels, and a subcervical lesion. This scoring system had a sensitivity of 85% and a specificity of 97% for the diagnosis of SDAVF on spinal angiography. Most interesting, all patients in our study met at least 3 of these criteria.
Delayed diagnosis and treatment of SDAVFs are associated with a very poor prognosis. Iovtchev et al2 reported a series 7 patients with delayed (60–730 days) diagnosis of SDAVFs. All 7 patients had become wheelchair-bound by the time of treatment, and 4 remained nonambulatory after treatment of the fistula and rehabilitation. Cenzato et al17 reviewed 65 patients with SDAVFs undergoing surgical and endovascular treatment and found that patients with the best clinical outcomes were those who had been diagnosed early and had an Aminoff scale score of <3 before the intervention. While delayed diagnosis was not an independent predictor of poor postoperative outcome in the Cenzato series, it was associated with a higher degree of disability at the time of treatment and thus a poorer clinical outcome following treatment.17 A previous analysis of 153 patients with SDAVF treated surgically at our institution did not find an independent association between the time from symptom onset to fistula treatment and postoperative prognosis; however, preoperative disability was the strongest determinant of postoperative outcome.18 In the current study focused on patients with delayed diagnosis, treatment very often took place only once substantial disability had already developed, and all too frequently this disability proved irreversible despite successful obliteration of the fistula.
Recommendations
Our study and others highlight the importance of timely diagnosis of spinal dural arteriovenous fistulas. Because most of these patients will only receive imaging of the lumbar spine without contrast during the initial evaluation of their symptoms, often the only sign of an SDAVF will be a slightly increased signal in the conus with some flow voids. These “edge of the film” findings are commonly missed, highlighting the necessity for the radiologist to specifically examine the conus in every lumbar spine MR imaging. In cases of patients with unexplained myelopathy, a spine MRA should be considered. Spine MRA has a high sensitivity for detection of SDAVFs as does 64–detector row multidetector CTA.19,20 In cases in which there is a high clinical suspicion, careful conventional spinal angiography should be performed because it is both safe and the criterion standard for detection of SDAVFs.21 In cases in which an artery cannot be accessed or assessed on the first attempt, there should be a low threshold for repeat angiography at a later date to ensure that all vessels are fully evaluated.
Limitations
Our study has limitations. First, most patients included in this study had extensive imaging evaluations performed before referral to our institution. Only a small proportion came with outside imaging reports; thus, we were unable to determine whether the signs of an SDAVF were mentioned in the radiology report and just not followed up clinically or if they were missed or misinterpreted, resulting in a delay in diagnosis. However, we can be certain that these patients were at least clinically misdiagnosed because only a small proportion ever underwent spinal angiography, and of those who did, all were interpreted as having negative findings. It is possible that the physician taking care of the patient disregarded the imaging diagnosis and treated the patient incorrectly or that patients may have initially refused spinal angiography.
Another limitation is the risk of bias in retrospectively interpreting the initial diagnostic imaging because both reviewers were aware that an SDAVF was present. However, the purpose of this study was not to evaluate the sensitivity of radiologists in detecting SDAVFs; rather, the purpose was to determine the clinical outcomes of those who had characteristic imaging findings but had a delay in diagnosis. We acknowledge that in many cases, the imaging findings could be subtle (ie, subtle flow voids or subtle T2 signal hyperintensity); however, in >80% of cases, patients had high T2 cord signal intensity and characteristic T2 flow voids, both characteristics that are highly suggestive of the SDAVF diagnosis. Another limitation is that we retrospectively determined the Aminoff motor disability and mRS scores through chart review. We may have underestimated or overestimated mRS scores, especially those in the 1–3 range. The Aminoff motor disability score, however, is easier to assess in a retrospective chart review because most of the necessary data can be easily abstracted from the documented physical examination (ie, use of a cane, walker or wheelchair, gait instability, and so forth). Last, because ours is a large referral center, there is definitely a risk of referral bias.
Conclusions
Delayed diagnosis of SDAVFs frequently leads to unnecessary pharmacologic and surgical treatments. Delayed diagnosis of SDAVFs in patients with imaging features of the disease results in high rates of additional morbidity, which is often irreversible despite successful treatment of the fistula. Thus, timely diagnosis of these lesions is essential to avoid additional morbidity from worsening of the myelopathy. Clinicians should have a low threshold for performing noninvasive angiography in patients with unexplained myelopathy.
Footnotes
Disclosures: Alejandro A. Rabinstein—UNRELATED: DJO Global,* Comments: for an investigator-initiated project on upper limb deep venous thrombosis prevention; Royalties: Elsevier,* Oxford,* Comments: for authored books. Giuseppe Lanzino—UNRELATED: Consultancy: Covidien.* *Money paid to the institution.
References
- Received May 24, 2015.
- Accepted after revision June 30, 2015.
- © 2016 by American Journal of Neuroradiology