Abstract
BACKGROUND AND PURPOSE: Following long-term spaceflight, a subset of the National Aeronautics and Space Administration astronauts present with visual impairment and increased intracranial pressure, known as visual impairment and intracranial pressure syndrome. We investigated structural brain changes following long-term head-down tilt bed rest as a spaceflight analog.
MATERIALS AND METHODS: Volumetric analysis was performed on structural pre- and post–bed rest brain MR images.
RESULTS: Comparing post–bed rest to pre–bed rest images, we found the following: 1) no significant group differences in GM, WM, CSF, or ventricular volumes; 2) shift of the center of mass of the brain upward and posterior rotation of the brain relative to the skull; 3) a significant correlation between posterior brain rotation and changes in ventricular volume; and 4) significant increases in brain tissue density in regions at the vertex, including the frontoparietal lobes, with contraction of adjacent extra-axial CSF spaces, and significant decreases in tissue density in areas along the base of the brain, including the orbitofrontal cortex.
CONCLUSIONS: We observed widespread morphologic changes with brain tissue redistribution in response to gravity changes; possible associated functional changes are unknown. The observation that ventricular change is correlated to posterior brain rotation suggests an alteration in CSF homeostasis. Ultimately, to elucidate any structural changes that may play a role in visual impairment and intracranial pressure syndrome, volumetric analysis of pre- and postflight structural scans of astronauts is needed.
ABBREVIATIONS:
- BR
- bed rest
- ICV
- intracranial volume
- IIH
- idiopathic intracranial hypertension
- NASA
- National Aeronautics and Space Administration
- VIIP
- visual impairment and intracranial pressure
Following long-term missions aboard the International Space Station, increased intracranial pressure and papilledema have been documented in the National Aeronautics and Space Administration (NASA) astronauts. In 1 report1 investigating 7 astronauts following 6 months of spaceflight, all astronauts demonstrated ophthalmologic findings, with disc edema in 5 astronauts and globe flattening in 5. Lumbar punctures were performed in 4 of these astronauts with opening pressures of 21–28.5 cm H2O1. In the 1 astronaut who underwent repeated lumbar punctures, the opening pressure remained elevated 19 months following spaceflight at 22 cm H2O1. The etiology of these findings is currently unclear; however, it has been hypothesized that they may result from loss of gravitational hydrostatic pressure gradients and large cephalad fluid shifts. NASA has coined the term “visual impairment and intracranial pressure [VIIP] syndrome” to describe this constellation of signs and symptoms in astronauts and has likened VIIP syndrome to Earth-based idiopathic intracranial hypertension (IIH) or pseudotumor cerebri.
A traditional ground-based analog used by NASA and other international space agencies to study physiologic changes associated with long-term spaceflight has been to place healthy subjects in 6° head-down tilt bed rest for varying periods.2,3 Anecdotally, Russian scientists first devised the head-down-tilt protocol in the early 1970s on the basis of reports by Russian cosmonauts who had the sensation of slipping off the foot of the bed on return to Earth after long-duration missions.3 The foot of the bed was raised until it felt horizontal to help the cosmonauts sleep.3 As an analog for spaceflight, the reduction in Gz gravitational stimuli during bed rest results in an upward shift of body fluids, unloading the upright weight of the body, reduced work against the force of gravity, and lower extremity inactivity.3 As a result, many of the physiologic changes of spaceflight can be reproduced, including decreased cardiac output, orthostatic intolerance, muscle atrophy, and bone loss. This model has been applied extensively to investigate cardiovascular and musculoskeletal deconditioning, immunologic response, and cognitive functioning.2,3
We previously acquired structural MR imaging brain scans of subjects participating in a NASA-sponsored long-term bed rest study.4 Given the recent interest in intracranial adaptation to spaceflight, we decided to perform a volumetric analysis of the structural MR imaging dataset to assess any potential alterations in brain structure or CSF distribution that may shed light on the spectrum of findings noted in VIIP syndrome. The results of this analysis are presented here.
Materials and Methods
Bed Rest Study Protocol
Eight healthy volunteers (age, 33 ± 7.4 years; 3 women) underwent long-term bed rest at the NASA Flight Analogs Facility at the University of Texas Medical Branch, Galveston, as part of a multi-investigator study.2 Informed consent was obtained, and the study was approved by the appropriate institutional review boards. Subject recruitment included radio, television, and newspaper announcements and a Web site (www.bedreststudy.com). The study protocol has been described in detail elsewhere.2 Before bed rest, subjects were admitted to the NASA Flight Analogs Facility and underwent a NASA-modified Air Force Class III physical examination and psychological assessment. Subjects then remained in the unit for 11–14 days before the start of bed rest for pre–bed rest data collection performed by other investigators and were free to ambulate normally. Male subjects were admitted into the study just after providing informed consent. Women were admitted so that the time of their next menses would occur 2 days before entering the bed rest phase of the study.2 For ease of reference, study days during the pre–bed rest period are referred to as BR-x, with BR-1 being the last day before bed rest. The first day in BR is referred to as BR1. After bed rest following return to normal ambulation, subjects remained in the unit for 2 weeks to undergo reconditioning.
Throughout the bed rest portion of the study, the subjects' beds were placed in a 6° Trendelenburg position. Subjects were allowed to lift their heads on 1 elbow to eat. Otherwise, the head-down position was strictly maintained, though the subjects were free to move about in their beds and shift from supine to lateral or prone positions. Bedpans were used, and hygiene was maintained with overhead showers while remaining in the head-down position. To keep actively engaged, subjects participated in various cognitive tasks, completed a specified objective (such as learning a foreign language), and were provided with numerous entertainment opportunities (movies, video games, group social activities). The subjects were placed on a standardized diet so that all input and output were maintained to ensure balanced nutritional uptake and hydration with no net water gain or loss.2 Monitors were present 24/7 to ensure subject compliance.
Initial plans were for all subjects to remain at bed rest for 90 days, and the first 4 subjects followed this protocol. The second group of subjects, unfortunately, was removed from the Flight Analogs Facility earlier than planned due to extreme weather. These subjects had undergone 42, 44, 49, and 52 days of bed rest at the time of the evacuation.
MR Imaging
Two brain MR imaging structural scans were obtained for each subject: one immediately before bed rest (pre–bed rest scan) and another toward the end of bed rest (referred to as the post–bed rest scan). On the first day of bed rest, BR1, the subjects walked to the MR imaging scanner facility and laid down on the MR imaging table for acquisition of the pre–bed rest scans. Immediately on completion of the MR imaging session, the subjects were moved from the scanner table while remaining supine and placed in a hospital bed in the 6° head-down-tilt position. Subjects then remained in this position throughout the bed rest period. For the 4 subjects who required emergent evacuation from the Flight Analogs Facility, end-of–bed rest imaging was performed on the day of evacuation, (ie, on days BR42, BR44, BR49, and BR52). On that day, while still in bed rest, the subjects were moved from their beds onto the MR imaging table, only shifting from head-down-tilt to supine. Structural scans were immediately acquired, and these were considered the post–bed rest scans. Following the scanning session, subjects were allowed to sit up and prepare for evacuation. For the 4 subjects who underwent 90 days of bed rest, MR imaging was performed on both day BR60, with the subjects remaining in the supine position only shifting from their bed to the scanner table after which they continued on bed rest for another 30 days, and day BR90. These subjects were not allowed to return to ambulation until BR90; however, given the shortened bed rest duration experienced by the evacuation subjects, for comparison purposes, in this report, the BR60 scans were considered the post–bed rest scans for these subjects. Therefore, considering all 8 subjects, the post–bed rest scans described in this report were obtained between days BR42 and BR60.
The structural MR images consisted of T1-weighted 3D echo-spoiled gradient-echo images acquired on a 1.5T scanner (GE Healthcare, Milwaukee, Wisconsin). Images were acquired in either transverse or coronal planes, at either 1.0- or 1.5-mm section thickness with FOVs of 250 or 260 mm. Scanning parameters were TR = 22 ms, TE = 8 ms, flip angle = 30°, and matrix = 256 × 256.
Physiologic Data
Because this study was part of a larger NASA-sponsored multi-investigator study, extensive physiologic data were obtained. With institutional review board approval, other investigators provided the following parameters: daily water intake, daily urine output, plasma volume, blood volume, and urine cortisol levels. The acquisition of these data points are described by the relevant investigators.2,5,6
MR Imaging Analysis
Voxel-Based Morphometry Analysis.
Data preprocessing and analysis were performed with the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) incorporated in the SPM8 software (http://www.fil.ion.ucl.ac.uk/spm/software/spm8) by using the default longitudinal preprocessing approach. We performed the following preprocessing steps: 1) registration of the post–bed rest scan to the pre–bed rest scan for each subject separately, 2) intrasubject bias correction, 3) segmentation of the different tissue classes, and 4) linear (ie, affine) and nonlinear (ie, Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra) registration. The segmentation procedure was refined by accounting for partial volume effects, applying adaptive maximum a posteriori estimations, and applying a hidden Markov random field model. The realigned and normalized GM and WM segments were smoothed with an 8-mm full width at half maximum Gaussian kernel. The images were then masked by a brain tissue mask defined by the probability of GM + WM > 80%.
Intracranial Volume Estimation.
Manual measurements were performed on the T1-weighted images from the pre–bed rest and post–bed rest scans. Twelve delineations were performed by 1 tracer (E.W.D.), and the other 4, by a second tracer (X.Z.). Details of the measurement of intracranial volume (ICV) are described elsewhere.7 In brief, the original sections were reformatted into the sagittal plane. Realigning the original sections to correct for head tilt was not necessary due to the large size of the intracranial cavity.8 The brightness of the image was increased to improve the visual clarity of the boundary of the dura mater. Starting from the left-hand side of the head, we then estimated the ICV for each image by a hand-traced mask. The tracers were blinded as to which subject/time point the scans represented. The 2 tracers reviewed each other's ICV definitions for consistency, and no significant difference was found between the 2 tracers (P = .25).
Tissue-Specific Volumetric Analysis.
GM, WM, and total intracranial CSF volume estimation was performed with the default “read raw volumes” function in the VBM8 toolbox. All images were segmented by using default templates (a modified version of the ICBM Tissue Probabilistic Atlas, http://www.bmap.ucla.edu/portfolio/atlases/ICBM_Probabilistic_Atlases/) and parameters. Specifically, these parameters included 2 Gaussians each for WM, GM, and CSF and 4 Gaussians for everything not fitting these categories; a warping regularization value of 1; a warp frequency cutoff of 25; very light regularization (0.0001); a 60-mm cutoff for the full width at half maximum of Gaussian smoothness of bias; and a sampling distance of 3. When generating masks, voxels with a probability of ≥0.5 were counted as members of that particular class.
Ventricular volumetric analysis was performed in a completely automated manner via FreeSurfer (http://surfer.nmr.mgh.harvard.edu). Standard reconstruction procedures, which delineate gross brain anatomy into a series of cortical and subcortical labels, were used. Structures are labeled by using a complex algorithm combining information on image intensity, probabilistic atlas location, and the local spatial relationships between subcortical structures by the FreeSurfer “recon-all” function.9 Ventricular volume was defined as the sum of the lateral ventricles (including the choroid plexus) and the third and fourth ventricles. The GM, WM, total intracranial CSF, and ventricular volumes were compared pair-wise by using paired t tests.
Brain-Shift Analysis.
To compare brain movement in reference to the skull, the skull was stripped from the images and used as a reference to calculate parameters for rotation and translation to estimate the difference before and after bed rest by using the FLIRT tool (FMRIB Linear Image Registration Tool, http://www.fmrib.ox.ac.uk/) of FSL. Because some of the images were originally acquired in the coronal plane, each pair of images (pre- and post–bed rest scans) was first resampled to the transverse plane. The skull image, obtained by using the ICV mask to remove all intracranial voxels, and the brain image, which included all voxels in the ICV mask, were then generated. The skull image of the pre–bed rest scan was then used as reference for a 6 df registration, to calculate the transformation matrix needed to align the post–bed rest skull to the reference. The same transformation matrix was then applied to the post–bed rest brain image to create the skull-aligned post–bed rest brains. Finally, the transformation matrix for aligning the post–bed rest brain to the pre–bed rest brain image was estimated on the basis of rigid-body assumptions, and the transformation/rotation parameters were calculated (On-line Fig 1).
Results
On visual inspection by an experienced neuroradiologist (D.R.R.), comparing pre–bed rest with post–bed rest images, a clear change in brain structures was seen. The extra-axial CSF spaces near the vertex appeared more crowded, and the CSF spaces along the frontal lobes were more prominent on the post–bed rest images compared with the pre–bed rest images (Fig 1). The supravermian cistern appeared effaced on the post–bed rest images. Additionally, a noticeable change in ventricular size was seen in some individuals. Most interesting, these findings varied across individuals: In some subjects, the ventricles became visibly smaller, while in others the ventricles became larger (Fig 2). On the basis of the subjective findings and the fact that the subjects had been maintained in the head-down-tilt position, we hypothesized that there would be a global shift in brain tissue within the cranial vault toward the vertex.
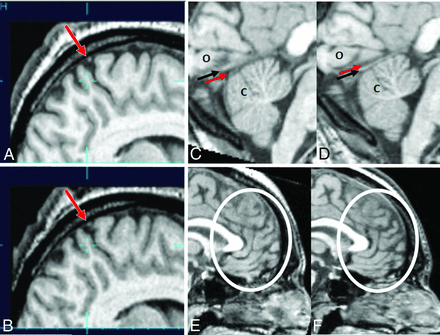
Structural images before and after bed rest. Sagittal images of the brain (subject H in On-line Tables 1–4) at the vertex before (A) and after (B) bed rest show a shift of the brain toward the vertex with contraction of the extra-axial spaces at the vertex and crowding of adjacent structures, including a cortical vein (arrow). Sagittal images of the posterior fossa before (C) and after (D) bed rest (subject A in On-line Tables 1–4). Before bed rest, the occipital lobes (o) lie against the tentorium cerebelli (black arrow). Below the tentorium, there is a thin layer of CSF (red arrow) between the tentorium and the upper aspect of the cerebellum (c). After bed rest, there is an upward shift of the occipital lobes away from the tentorium, now with a thin layer of CSF (red arrow) between the occipital lobes and the tentorium. In the posterior fossa, a layer of CSF is no longer visible between the tentorium and the cerebellum. Instead, the cerebellum now appears compressed against the tentorium. Sagittal images of the frontal lobes before (E) and after (F) bed rest show subtle expansion of the frontal lobe sulci after bed rest (subject A in On-line Tables 1–4).
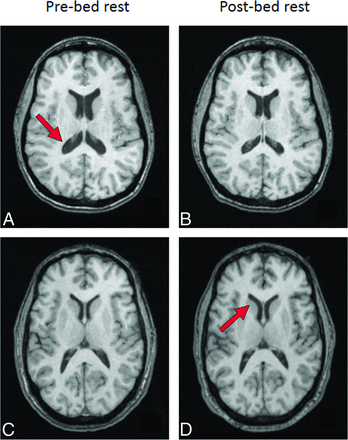
Changes in ventricular volume before and after bed rest. Axial images of the brain of the subject with the largest change in ventricular size on the post–bed rest scan (B) compared with the pre–bed rest scan (A). Compared with pre–bed rest, there was a 22.4% reduction in ventricular size post–bed rest in this subject, best appreciated at the level of the atrium of the lateral ventricles (arrow) (subject D in On-line Tables 1–4). Axial images of the brain of the subject with the largest increase in ventricular size on the post–bed rest scan (D) compared with the pre–bed rest scan (C). Compared with pre–bed rest, there was a 10.4% increase in ventricular size post–bed rest in this subject, best appreciated at the level of the frontal horns of the lateral ventricles (arrow) (subject C in On-line Tables 1–4).
Tissue-Specific Volumetric Analysis
On the group level, the GM, WM, CSF, and ventricular size did not demonstrate significant differences (P > .1) between pre- and post–bed rest (Table 1, See On-line Tables 1–4 for individual subject data). This held true for both the raw and normalized (tissue volume/estimated ICV) volumes. Although by visual inspection, a distinct change in ventricular volume was seen for some subjects between pre- and post–bed rest, on group analysis, this did not reach statistical significance due to the variability of responses across individuals (On-line Fig 2). No correlation was noted between sex and ventricular volume change.
Pre- and post–bed rest volumetric MRI and physiologic measurements (group mean and SD)
Brain-Shift Analysis
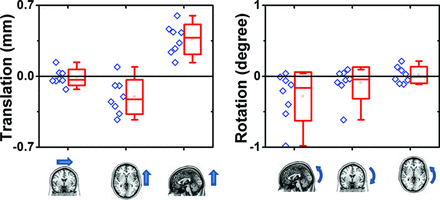
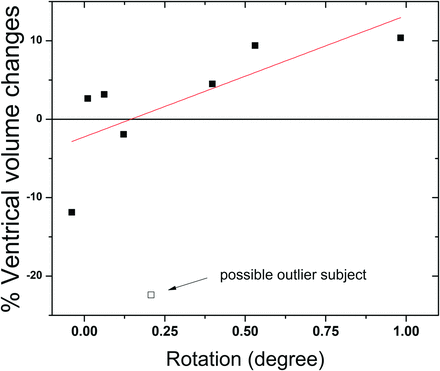
After bed rest, the brain as a whole showed significant displacement in the inferior-to-superior direction (0.36 ± 0.15 mm, t = 7.0, P < .01), and marginally significant displacement in the anterior-to-posterior direction (0.21 ± 0.17 mm, t = 3.4, P = .11), with no significant effect in the left-right direction (0.01 ± 0.08 mm, t = 0.4, P = .69) (Fig 3). Rotation was found to be significant around the left-right axis (0.28 ± 0.34°, t = 2.3, P = .051), but not around any other axis (P = .27 and 0.69). In other words, the center of mass of the brain was shifted upward, and the brain demonstrated posterior rotation relative to the skull. The change in ventricular size was found to correlate (Spearman correlation: r = 0.893, P = .007) with the degree of posterior rotation of the brain independent of subject variability (Fig 4).
Brain translation and rotation in reference to the skull following bed rest. Parameters (translation and rotation on x, y, z-axes) were estimated on the basis of a rigid-body assumption. The arrow indicates the direction of movement that corresponds to positive values on the graph.
Correlation between brain rotation and ventricle volume changes. Without the potential outlier, which was the subject that demonstrated the largest ventricles before bed rest, the Spearman correlation is r = 0.893, P = .007. Including the outlier, the Spearman correlation is r = 0.690, P = .058.
Voxel-Based Morphometry
Paired t tests were used to estimate the possible changes of GM or WM between pre- and post–bed rest scans. The predictions were compared and thresholded at P = .001 (t = 3.1) and a cluster size of >296 voxels. Compared with pre–bed rest, the post–bed rest brains showed significant tissue (GM + WM) density increase in regions near the vertex, including the central frontoparietal lobes (Fig 5, See On-line Fig 3 for more detail). Other regions that also showed significant density increase included the posterior cingulate, cuneus, thalami, and cerebellum. Tissue-density decrease was found in the orbitofrontal cortex, brain stem, corpus callosum, striatum, anterior cingulate, and parietal operculum.
Regions of the brain most significantly affected by bed rest. There is increased brain tissue density (top row of images) at the vertex, particularly affecting the central frontoparietal lobes, with contraction of the adjacent CSF spaces (bottom row of images). There is decreased brain tissue density along the base of the brain, including the orbitofrontal cortex, with expansion of the adjacent CSF spaces.
Physiologic Data
The Spearman rank correlation was used to assess the association between physiologic data (fluid balance, plasma volume, blood volume, and cortisol levels provided by other investigators5,6) and MR imaging measures. No significant correlation was found (Table 1). See On-line Tables 1–4 for individual subject data.
Discussion
NASA has recently described a constellation of findings, including visual decrement and elevated intracranial pressure, occurring in a subset of astronauts following long-term exposure to microgravity and the space environment, known as the VIIP syndrome. These are especially concerning given that plans for human spaceflight, such as a manned Mars mission, will be of even longer durations than current assignments on the International Space Station.
As a first step in elucidating the mechanisms underlying the development of the VIIP syndrome, we examined structural brain changes induced by long-term 6° head-down tilt bed rest, a traditional NASA model for studying physiologic changes associated with microgravity exposure. The consequences of long-term maintenance of a head-down body position on the brain are unknown; however, changes in body position and resultant changes in gravitational gradients have been found to impact brain physiology. For example, moving from the upright to the supine position alters venous outflow, with redirection of venous return from the vertebral venous plexuses to the internal jugular veins; decreases intracranial compliance; and increases intracranial pressure.10 In this study, we found the following: 1) no significant group differences in GM, WM, CSF, or ventricular volumes between pre- and post–bed rest; 2) shift of the center of mass of the brain upward and posterior rotation of the brain relative to the skull; 3) significant correlation between posterior brain rotation and changes in ventricular volume; and 4) significant increases in brain tissue density in brain regions at the vertex, including the central frontoparietal lobes, with associated contraction of the adjacent extra-axial CSF spaces and significant decreases in tissue density in areas along the base of the brain, including the orbitofrontal cortex, with expansion of the basal extra-axial CSF spaces.
We hypothesize that these unique structural alterations occurring during bed rest and possibly during spaceflight due to altered gravity gradients may provide explanations for the findings of the VIIP syndrome. The extra-axial CSF effacement at the vertex found during bed rest may lead to changes in CSF flow dynamics, and an upward shift of the brain may result in compression of the dural venous sinuses along the vertex. This, in turn, may contribute to venous outflow obstruction and the spectrum of intraorbital and intracranial findings described in astronauts. This hypothesis is further supported by the observation that ventricular change is correlated to posterior brain rotation within the skull, suggesting altered CSF homeostasis. Future planned studies include advanced structural, functional, and venographic MR imaging of astronauts before and after spaceflight.
In some subjects in our study, the ventricular volume change is clearly visible without computer-aided analysis (Fig 2). The change in ventricular size ranged from a 10.4% increase to a 22.4% decrease post–bed rest compared with pre–bed rest values, an unexpected finding during the course of only 2 months in young, healthy subjects. For comparison, in elderly, healthy subjects, expansion of the ventricles has been reported to be 1.5%–3.0% per year and 5%–16% in patients with Alzheimer disease.11 Ventricular volume can also be influenced by fluid balance, and the subjects with bed rest did undergo an initial diuresis, which stabilized within 3 days.2 However, ventricular volume changes would be expected to be approximately 1%–3% during dehydration relative to normal hydration,12 again less than the changes in ventricular size that we measured.
It has been suggested that the spectrum of intraorbital and intracranial findings of astronauts following spaceflight are similar to those in the Earth-based condition of IIH.1,13 Increased intracranial pressure, papilledema, globe flattening, dilated optic nerve sheaths, and visual disturbances have been described in both conditions (Table 2).1,13,14 Although the etiologies of both conditions are unknown, researchers speculate that venous insufficiency or hypertension caused by cephalad fluid shifts during spaceflight are possible mechanisms for the VIIP syndrome in astronauts. Similarly, the pathophysiology of IIH is thought to be related to decreased CSF absorption at the arachnoid villi possibly due to venous-outflow obstruction. However, there are important clinical differences between the VIIP syndrome and IIH. In the VIIP syndrome, none of the astronauts with ocular changes presented with clinical symptoms typical of IIH, including chronic headaches, diplopia, or transient visual obscurations.1 Moreover, while IIH is typically a syndrome of female patients with obesity,14 VIIP is more common in male astronauts.1
Comparison of typical clinical features in IIH and VIIP
Similarly, in our study of structural brain changes associated with long-term bed rest, we found important structural differences in our subjects from those previously described in patients with IIH. For example, in a volumetric MR imaging study of patients with IIH,15 a significant increase in extraventricular CSF volume was found, particularly along the vertex. Following bed rest, we found instead effacement of the extraventricular CSF spaces along the vertex and crowding of adjacent brain tissue, including the frontoparietal lobes. Other investigators have suggested an overlap in findings between patients with IIH and those with Chiari I malformation, with an incidence of 10%–21% of cerebellar tonsillar ectopia of >5 mm in patients with IIH.16 In our study of subjects with bed rest, we demonstrated the opposite finding: a shift of the brain tissue upward in the skull with decreased crowding at the foramen magnum. If the head-down tilt bed rest model is an appropriate analog for the intracranial changes experienced by astronauts, then the Earth-based IIH syndrome may not be an appropriate model for VIIP syndrome. A volumetric analysis of the structural brain imaging data of astronauts would be informative in this respect.
Unrelated to VIIP symptoms and CSF homeostasis, morphologic changes were observed affecting widespread regions of the brain, with the frontal and parietal lobes (areas of the brain associated with sensorimotor and high-level cognitive functions) demonstrating the most prominent changes. The consequences of structural changes affecting functional brain areas is unknown but may be crucial given the involvement of these areas in adaptation to microgravity. For example, optimal performance in the microgravity environment requires re-interpretation of sensory input from peripheral receptors.17 Because the lower extremities are used less for locomotion, new sensorimotor strategies emerge in spaceflight, requiring plasticity at higher cortical levels, including the sensorimotor cortices.17 Disturbances in perceptual-motor task performance have been found during spaceflight, particularly while astronauts simultaneously engage in secondary cognitive tasks.17 These findings warrant further investigation, including planned and ongoing functional brain imaging studies of astronauts following long-term spaceflight.
Our study has several limitations. Definite conclusions concerning the VIIP syndrome cannot be made because this study was performed in subjects with bed rest and not the astronaut population. Most important, the MR imaging protocol was not initially designed to address structural changes in bed rest, and volumetric analysis was only undertaken much later after experiment completion to address the recent interest in intracranial adaptation to spaceflight. Therefore, the MR imaging protocol was not optimized for structural analysis with no datasets from control subjects to demonstrate between-session reproducibility. However, other investigators have demonstrated that volumes derived from automated segmentation of T1-weighted structural images are reliable measures when performed on the same scanner platform, finding the variability in volume across sessions to be <2.3% for subjects of a similar age group as in our study.18 The influence of sex hormones and the menstrual cycle may also play a role in the structural brain changes seen in bed rest. Although, data concerning sex hormones were not available, the 3 female subjects in this study were admitted to bed rest in a standardized fashion related to the time of menses. Furthermore, no significant correlation was found between volumetric changes and sex, at odds with the male predominance in VIIP syndrome and the female predominance of IIH.
Conclusions
The consequences of long-term microgravity exposure on the brain are poorly understood but may include alterations in brain structure, vasculature, and function, leading to the VIIP syndrome experienced by some astronauts. In this study, we used long-term bed rest as an analog for spaceflight, to study structural changes of the human brain. Our results indicate that the brain as a whole can move within the skull in response to gravity changes, and in the head-down tilt bed rest model, the brain moved upward and rotated posteriorly in relation to the skull. There was a significant correlation between brain movement and changes in ventricular volume, suggesting altered CSF homeostasis and a possible explanation for the constellation of VIIP symptoms. Unrelated to the VIIP syndrome, widespread morphologic changes of the brain were observed as a result of brain tissue redistribution in response to gravity changes. These locally occurring morphologic changes may lead to possible brain function changes. For example, the frontal and parietal lobes were most affected, which could affect sensorimotor and high-level cognitive functions. Ultimately, to elucidate any structural changes that may play a role in VIIP syndrome, a volumetric analysis of the structural scans of astronauts before and following long-duration spaceflight is needed. The findings of this study may also be relevant to Earth-based, chronically ill patients who are confined to long-term bed rest.
ACKNOWLEDGMENTS
The authors wish to thank Dr Satish Mehta for providing cortisol data. Other physiological data was obtained from the NASA Life Sciences Data Archive.
Footnotes
Disclosures: Donna R. Roberts—RELATED: Grant: NASA*; Support for Travel to Meetings for the Study or Other Purposes: NASA; UNRELATED: Grants/Grants Pending: NASA, Comments: I am currently the Principal Investigator of a NASA grant separate from the one that funded the research described in the article. Edward W. Duffy—RELATED: Grant: Palmetto Academy. David A. Ramsey—RELATED: Grant: NASA,* Comments: NASA funded the study at MUSC who subcontracted my employer at the time (SCRA); Support for Travel to Meetings for the Study or Other Purposes: NASA,* Comments: As above. Truman R. Brown—UNRELATED: Grants/Grants Pending: NASA,* Comments: grant to study possible brain shifts after space flight. *Money paid to the institution.
This work was funded by NASA, grant number NNJ04HF70G.
The authors wish to dedicate this article to Ali Tabesh, PhD.
Paper previously presented at: American Society of Neuroradiology and the Foundation of the ASNR Symposium, May 17–22, 2014; Montreal, Quebec, Canada.
Indicates open access to non-subscribers at www.ajnr.org
REFERENCES
- Received February 5, 2015.
- Accepted after revision March 4, 2015.
- © 2015 by American Journal of Neuroradiology