Abstract
BACKGROUND AND PURPOSE: The relationship between enlarged subarachnoid spaces and subdural collections is poorly understood and creates challenges for clinicians investigating the etiology of subdural collections. The purpose of this study was to determine the prevalence of subdural collections on cross sectional imaging in children with macrocephaly correlating with subarachnoid space enlargement.
MATERIALS AND METHODS: The radiology information system of a large pediatric medical center was reviewed for “macrocrania” and “macrocephaly” on reports of cranial MRI/CT examinations in children <24 months of age, over a 24-month period. Head circumference was obtained from the clinical record. Studies were reviewed blindly for subdural collection presence and subarachnoid space size. Children with prior cranial surgery, parenchymal abnormalities, hydrocephalus, or conditions predisposing to parenchymal volume loss were excluded. Chart review was performed on those with subdural collections.
RESULTS: Imaging from 177 children with enlarged head circumference was reviewed. Nine were excluded, for a final cohort of 168 subjects (108 with enlarged subarachnoid space). Subdural collections were identified in 6 (3.6%), all with enlarged subarachnoid space (6/108, 5.6%). In 4, subdural collections were small, homogeneous, and nonhemorrhagic. In 2, the collections were complex (septations or hemorrhage). Two children were reported as victims of child abuse (both with complex collections). No definitive etiology was established in the other cases.
CONCLUSIONS: The prevalence of subdural collections in imaged children with macrocrania was 3.6%, all occurring in children with enlarged subarachnoid space. Our results suggest that enlarged subarachnoid space can be associated with some subdural collections in this cohort. Despite this, we believe that unexpected subdural collections in children should receive close clinical evaluation for underlying causes, including abusive head trauma.
ABBREVIATIONS:
- SS
- subarachnoid spaces
- BESS
- benign enlargement of the subarachnoid spaces
- SDH
- subdural hemorrhage
- SDC
- subdural collections
- HC
- head circumference
- AHT
- abusive head trauma
- CAT
- child abuse team
Enlargement of the subarachnoid spaces (SS) is a common finding in children undergoing cranial imaging evaluation for various clinical conditions. In the setting of large or rapidly growing head circumference (HC), normal or mildly enlarged ventricles with enlargement of the SS, particularly over the frontal lobes, these children are usually diagnosed with “benign enlargement of the subarachnoid spaces” (BESS).1⇓⇓⇓–5 Children with BESS typically have no neurologic or long-term developmental abnormalities with resolution of clinical and imaging findings within the first 2 years of life.6
Some investigators have suggested that children with macrocrania and enlargement of the SS may be at increased risk for subdural hemorrhage (SDH) after minimal or no trauma.7⇓⇓⇓⇓⇓⇓⇓–15 A clinical dilemma often arises when subdural collections (SDC) are identified in children with enlarged SS because the identification of SDC in an infant without an appropriate traumatic history raises the concern for abusive head trauma (AHT).3,12,16,17
The literature connecting enlarged SS and risk of SDC is based predominately on case reports or small case series. No current data are available on the prevalence of SDC in children with macrocrania and enlarged SS.8 The lack of scientific evidence for the relationship between enlarged SS and SDC creates uncertainty for clinicians in assessing for AHT. The purpose of this study was to determine the prevalence of SDC discovered on cross-sectional imaging in children with macrocrania during a defined time interval, correlating with SS enlargement.
Materials and Methods
This study was approved by the local institutional review board. The radiology information system of a large tertiary care academic pediatric medical center was reviewed for the period between January 1, 2008, through December 31, 2009, for the terms “macrocrania” and “macrocephaly” on reports of CT and MR imaging examinations of the head in children ≤24 months of age. Both CT and MR imaging were chosen to reflect clinical practice because these are both commonly ordered for concerns related to macrocrania. Children >24 months of age were excluded because BESS is typically much less prevalent after this time.2 Subjects with technically nondiagnostic examinations were excluded.
Only subjects with enlarged HC were included, defined as >95th percentile.18,19 To limit secondary causes of SS enlargement and SDC development, subjects were first excluded if they had brain tumors, prior intracranial surgery, shunts, craniosynostosis, prior intracranial hemorrhage, prior meningitis/abscess, or parenchymal abnormalities on imaging. Patients with ventricular enlargement out of proportion to cerebral sulcal prominence suggesting the possibility of hydrocephalus were also excluded.
CT imaging was performed with the use of 5-mm contiguous axial images. MRI included sagittal and axial T1-weighted, dual-echo axial FSE PD/T2, axial gradient recalled-echo, and coronal FSE T2-weighted images (5.0-mm section thickness, 1.0-mm gap). Each examination was reviewed by a board-certified radiologist with added qualification in neuroradiology and 18 years' experience interpreting neuroimaging (J.L.L.). Studies were reviewed in a blinded fashion for the presence and imaging characteristics of SDC and SS size (normal or enlarged). Differentiation of enlarged SS and SDC was made on the basis of standard imaging findings.3,16,20,21 Results were compared with the clinical imaging report, and discrepancies were reviewed by another board-certified radiologist with added qualification in pediatric radiology and a pediatric neuroradiology fellowship (M.C.). Remaining discrepancies were resolved by consensus opinion. For included cases, all subsequent imaging studies were reviewed.
A secondary exclusion was performed to exclude causes of brain volume loss that could promote development of secondary SDC, including prior chemotherapy, brain irradiation, failure to thrive or malnutrition, chronic systemic illness, chronic corticosteroid therapy, significant prematurity (GA <28 weeks), congenital anomalies, or brain atrophy pattern by imaging.22 All cases of identified SDC underwent a second chart review, to include dedicated retinal examination, skeletal survey, and child abuse team (CAT) reports.
Measurements of the SS were performed after qualitative assessment and after secondary exclusion. Measurements were made on axial CT images and axial T2-weighted MR images by use of a previously described technique.23 The largest distance of the frontal SS perpendicular to the calvaria was measured by a single blinded reviewer (J.L.L.) by use of the electronic ruler in the PACS. Measurements were made from the inner table to the cortex surface, on images in which the ventricles were visible. The largest value for each case was recorded. If an SDC was present, the collection was not included in the measurement of the SS.
Statistical analysis was performed by use of Fisher exact test for categoric data and t test for continuous data, with P < .05 deemed statistically significant.
Results
Two hundred seventy-nine CT/MR examinations fit initial study criteria. After initial exclusion, 242 cases (136 CT, 106 MR) formed the initial study group. Of these, 177 patients had an enlarged HC. Nine were excluded, for a final study group of 168 cases. Of the 168 cases, 108 had enlarged SS and 60 had normal SS. The final study group had an average age of 48.8 weeks (range, 9–101 weeks), with a predominance of boys (65%). The group with enlarged SS had a greater prevalence of boys (69%) compared with the normal SS group (52%) (P = .0298) and was younger (median age, 37.6 ± 17.2 weeks versus 51.6 ± 18.8 weeks, P < .00001).
Mean size of the frontal SS in children with qualitatively enlarged SS was 7.0 ± 2.0 mm (range, 4.1–14.6 mm). Mean size of the frontal SS in children with qualitatively normal SS was smaller, at 2.8 ± 1.1 mm (range, 1.1–5.6 mm) (P < .00001).
Six SDC were identified, for an overall prevalence of 3.6%. All 6 SDC were identified in subjects with enlarged SS, giving a prevalence in this group of 5.6% (6/108). No SDC were identified in the 60 patients with normal SS. The prevalence difference between groups approaches statistical significance (P = .06). The children with SDC were predominately boys (5:1), averaged 30.2 weeks of age, and were without reported recent accidental trauma (Table 1). Subjects with macrocrania and SDC were younger than those subjects without SDC (mean age, 30.2 weeks versus 40 weeks, respectively, P = .025).
Clinical presentation and follow-up in 6 children with enlarged subarachnoid spaces and subdural collections
The SDC were identified initially on CT in 2 cases and on MRI in 4 (On-line Table). All SDC cases were referred because of macrocrania. Both CT cases had MRI within 9 days, which confirmed the CT findings. SDC were bilateral in 2 cases and unilateral in 4, localized over the convexities, with a mean size of 5.4 mm (range, 1–9.6 mm). In 4 cases (cases 2, 3, 5, and 6), the SDC were homogeneous, unilateral, and small in size, with similar features on MRI (Fig 1). None of these subjects had recent (within 2 weeks) CT imaging for review. In 2 cases, the SDC were larger, bilateral, and complex in appearance. In case 4, there were heterogeneous signal changes on MRI and increased attenuation on CT, which suggests recent hemorrhage with identified membranes within the collections (Fig 2). In case 1, there was heterogeneous internal signal with visible membranes and different signal intensity components. No CT or gradient-echo evidence of acute hemorrhage was identified.
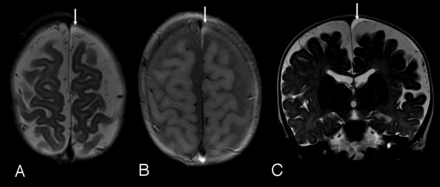
Case 3. A, Axial FSE T2-weighted image. B, Axial proton density–weighted image. C, Coronal FSE T2-weighted image; 8-month-old girl. Clinical indicatin for examination: macrocrania. Typical small homogeneous subdural collection, similar to those identified in cases 2, 5, and 6. Note diffuse prominence of subarachnoid spaces. Small left frontal vertex subdural collection is identified (arrows), slightly hyperintense to CSF on T2-weighted images (A and C), and moderately hyperintense to CSF on proton density images (B). The collection was isointense to CSF on T1-weighted images and showed no blooming on gradient-echo sequences. Follow-up CT 3 months later showed decrease in prominence of the subarachnoid space, normal ventricles, and no evidence of subdural collection.
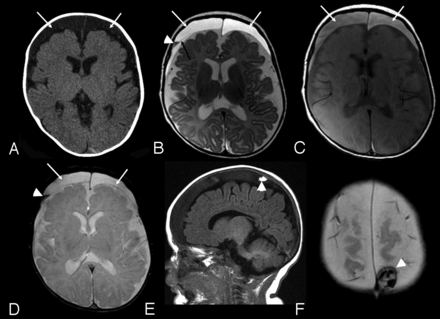
Case 4. A, Axial CT image. B, Axial FSE T2-weighted image. C, Axial proton density–weighted image. D, Axial gradient recalled-echo image. E, Sagittal T1-weighted image. F, Axial gradient-echo image: 3-month-old boy with macrocrania. Initial CT examination (A) demonstrates moderate sized bilateral subdural collections (arrows), slightly hyperattenuated relative to CSF. MRI examination was performed 14 hours later. Bilateral subdural collections are again identified (white arrows, B, C, and D) hyperintense to CSF on both T2 and proton density–weighted images (B and C). A thin septation is identified on the right (black arrow, B). A layering region of decreased T2 signal is seen on the right (arrowhead, B), which was hyperintense to CSF on T1-weighting (not shown) and blooms on the gradient-echo sequence (arrowhead, D) compatible with blood products. A localized area of increased signal on T1-weighted images in the right parietal vertex subdural collection was noted (arrowhead, E), which was hyperattenuated to brain on CT (not shown) and exhibited blooming on the gradient-echo sequence (arrowhead, F) consistent with additional blood products.
A multidisciplinary CAT consultation was requested for 2 of 6 cases with SDC (cases 1 and 4). Both had normal initial and follow-up skeletal surveys, and both had dedicated retinal examinations. In case 1, there were retinal hemorrhages characteristic for abuse. Two additional children with SDC had dedicated retinal examinations for hyperopia and astigmatism, and no retinal hemorrhages were seen. None had unexplained bruising. One child (case 6) with small, homogeneous, unilateral right-sided SDC had a history of a left-sided parietal skull fracture 3 months before the index examination. A CAT consultation at that time resulted in clinical findings consistent with accidental trauma. On CT examination at the time of skull fracture, the SS were noted to be prominent; however, no intracranial hemorrhage or SDC were identified. Ultimately, 2 children were reported to Children's Services as probable abuse, including the case with the retinal hemorrhages and complex SDC (case 1) and the case with the signal changes suggesting recent hemorrhage (case 4). The remaining 4 cases not reviewed by the CAT were not reported to Children's Services.
Four subjects with SDC had prior cranial imaging varying from the same day as the index study to 6 months previously. In case 3 (index MRI examination demonstrated small vertex SDC), a head sonography was performed 4 weeks previously. The SDC were not described on the original sonography report but were visible on retrospective review. In case 5, head sonography was performed 6 and 2 months before the index study and showed no evidence of SDC. In case 4, sonography was performed the same date as the index study, identifying bilateral SDC. In case 6, head CT was performed 4 months before the index MR imaging examination, with no evidence of SDC.
Follow-up imaging was performed in 5 cases from 3 weeks to 7 months after the index study. The SDC became smaller or resolved in each case with no intervention. In 1 case (case 4) 6 weeks after the index case showing bilateral SDC, a follow-up CT demonstrated smaller size, but with new increased attenuation within the right-sided collection. Repeat skeletal survey was negative. Clinical follow-up (range, 1–4 years) was available on 5 of 6 subjects. None of the children with SDC had a known bleeding disorder or genetic condition. None of the subjects required surgical drainage for treatment.
Discussion
Previous reports state that the most common cause of subdural hemorrhage in children is AHT.17,24 The finding of unexplained SDC in a young child should initiate consideration of AHT; however, this must be closely correlated with the clinical context, other imaging, and laboratory findings. Bleeding diatheses, various genetic conditions (eg, glutaric aciduria type I), overshunting, and rapid decreases in brain volume have been associated with SDC in children after minimal or no trauma3,16,25 and should be kept in mind when evaluating these patients.
Enlarged SS have been described in association with SDC in children by several authors and have been implicated as a predisposing cause.7,9⇓–11,20,26⇓⇓⇓⇓–31 Variable methodology, cohort makeup, clinical assessments for AHT, and imaging techniques in these studies make conclusions regarding SDC etiology difficult.
SDC in children can have a wide range of appearances, depending on etiology, imaging test used, and age of the collection.12,14,16 “Subdural hematoma” has been used very loosely in the literature in this population and probably has described a wide range of SDC with different etiologies, including “subdural hygroma,”25 “chronic subdural hematoma,”16 “chronic subdural hematoma with rebleeding,”12 and “hemato-hygroma.”14 In clinical practice, it is often difficult to differentiate these, adding to the complexity in evaluating these children.12,25 For this reason, we chose to use the nonetiologic term “subdural collections” in our study to describe these collections.
Five previous studies have described SDC in populations of children with enlarged SS with prevalence estimates between 8–23% in series with between 20–142 subjects. The largest of these studies described a mixed group of children with enlarged extra-axial spaces on head sonography.30 They found 33 SDC in 142 subjects over a 4-year period. Twenty-five were anechoic (CSF-like) and 8 were echogenic or complex; 103 of these subjects had macrocrania, though the presence of macrocrania and enlarged spaces was not correlated with SDC visualization. Although children with prior major hemorrhage were excluded, children with prior meningitic effusions, white matter injury of prematurity, and “malformative syndromes” were included in their cohort, limiting conclusions. Few subjects had CT imaging, limiting detection of acute hemorrhage. Of the children with SDC, 4 cases were “battered children”; however, AHT evaluation details are limited. Azais and Echenne29 describe 5 SDC in 41 children with “benign enlargement of the SS” identified primarily with sonography, 29 (72%) of whom had macrocrania. They excluded children with prematurity, intrauterine growth restriction, malnutrition, and neonatal distress. Three SDC were “unexpected” and not associated with any reported trauma. One was related to accidental trauma and 1 was related to probable AHT. Hellbusch26 described 9 SDC in 47 highly selected children referred for neurosurgical evaluation of enlarged extra-axial spaces. Traumatic cases (both accidental and AHT) were excluded, and no detailed description of imaging findings was reported.
Three recent studies specifically evaluated SDC in children with enlarged extra-axial spaces.10,11,32 However, because the cases were identified on the basis of the presence of SDC, no inference as to the true prevalence of SDC in the overall group with enlarged extra-axial spaces is possible. Ghosh and Ghosh10 described 9 patients (3 months to 2 years of age) with SDH and enlarged extra-axial spaces identified by searching for the ICD-9 diagnoses of “nontraumatic SDH” and “hydrocephalus” over a 10-year period. They excluded patients with true hydrocephalus, shunts, head injury, and cerebral atrophy. The identified collections were bilateral in 6 and exhibited a wide range of imaging appearances. All were evaluated for AHT, but only 1 subject was reported to children's services. McNeely et al11 described 7 children with SDC and enlarged SS identified in a nonspecified manner in a 6-year period. Children with known AHT and bleeding diatheses were excluded. Collections were bilateral in 3. One was involved in a major motor vehicle collision and 1 had a reported fall with skull fracture. The others were identified on imaging performed for macrocrania or lethargy, and no other etiology was identified. Vinchon et al32 described 16 children with “spontaneous” SDH (no traumatic history, bleeding disorder, or AHT) identified from a prospective neurosurgical data base; 12 had enlarged SS, and 12 had macrocrania. They compared patients with spontaneous SDH with a group with AHT or accidental trauma and found that the children with spontaneous SDH were much more commonly macrocephalic (75% versus 20%).
Although the published associations between enlarged SS and SDC as well as data from our study suggest a link, documented spontaneous SDC in children with enlarged SS and prior imaging without SDC, are rare. We could find only 12 previously reported spontaneous, nontraumatic SDC in children with prior imaging documentation.7,20,26,31⇓–33 The best documented of these reports is from Amodio et al,20 who described a 3.5-month-old, former 32-week preterm boy, with grade 1 germinal matrix hemorrhage presenting with macrocrania. Initial head ultrasound examination demonstrated enlarged extra-axial spaces but no SDC. With continuing HC increase, a 6-week follow-up head ultrasound examination was performed that demonstrated new bilateral SDC containing low-level echoes. A subsequent MRI demonstrated large bilateral SDC with layering hemorrhagic material, confirmed at surgical drainage. Evaluation for AHT (including skeletal survey and retinal examination) was negative. In evaluating the images in this report, marked diffuse subarachnoid space enlargement is noted, however, suggesting brain volume loss. In our study, 4 patients with SDC had previous imaging (ultrasound examination, 3; CT, 1). In 3 patients, SDC were not identified on prior imaging, though the size of the identified SDC probably would have been too small to visualize on the imaging test performed (ultrasound examination and CT). In 1 case, retrospective assessment of a prior head ultrasound examination documented small SDC, not mentioned on the clinical report.
Data from the current study and the limited literature do support that SDC may arise in children with enlarged SS, after minimal or no trauma. The exact etiology is incompletely understood. However, some theories have been postulated.12,14 Enlargement of the subarachnoid space may stretch and place increased strain on bridging veins, with resultant rupture and SDH formation.7,13,29 This potential increased strain on bridging veins has been modeled mathematically13 but is not universally accepted.34 Membranes surrounding an aging subdural hematoma are highly vascularized and have been postulated to potentially allow rebleeding either spontaneously or with minimal trauma.12,35,36 A rent in the arachnoid could occur, allowing communication of the subdural and SS and subsequent subdural CSF collections to form.14
Although numbers are small and the clinical child abuse directed assessment was not performed on all patients, our report suggests small homogeneous SDC on cross-sectional imaging, without definite evidence of hemorrhage, may occur in the setting of BESS and may not indicate inflicted injury. In our study, 2 of the 6 children with SDC had findings suggesting child abuse. Both of these subjects had moderate-sized, bilateral, heterogeneous collections. The remaining 4 patients had radiographically similar SDC that were small, homogeneous, and localized over the convexities. Heterogeneous, more complex collections, particularly with clear evidence of hemorrhage by CT or MRI, could represent an important finding that suggests inflicted injury in the appropriate clinical scenario.
Only a few of the previous studies of SDC in children with enlarged SS report documentation of a dedicated evaluation of AHT.7,8,10,20 In these studies, a total of 14 cases of SDC in the setting of enlarged SS have been described. All were investigated, but only 1 was thought to be related to AHT. Although imaging findings are incompletely described, 1 controversial case report describes a small “acute” SDH in a 4-month-old boy with macrocrania and enlarged extra-axial spaces who fell from a standing position to a carpeted floor.8 CT images were not presented in the report; however, MR images document small mixed intensity SDC. Although these studies document SDC in children after minimal or no trauma, they are all limited to varying degrees by incomplete description of imaging findings, variability in clinical assessment of potential abuse, and selection bias.
Age and sex differences were noted between subgroups of patients in our study. A male predominance in both the overall group of patients with macrocrania and those with enlarged SS was noted, consistent with prior studies.2 Subjects with macrocrania and enlarged SS were younger than those subjects with normal SS, a finding described in other studies of BESS.2 Enlarged SS typically normalize by 2–3 years of age in BESS.2,22,37 The patients with macrocrania and normal SS in our study may be at the later stages of this condition.
There are some limitations to this study. Although all subjects had enlarged HC and many (61%) had enlarged SS, how many of this group fulfilled the full clinical scenario of “benign enlargement of the subarachnoid spaces” is unknown. Given our extensive exclusionary process, most subjects with macrocrania and enlarged SS probably did fit the criteria for BESS. Evaluation of subarachnoid space size was subjective for categorization in our study but did rely on the assessments of at least 2 radiologists. Our subjective assessment was subsequently validated by a quantitative analysis. Prior studies of normative subarachnoid space size in children are few; however, findings on cross-sectional imaging and ultrasound examination23,38 correlate well with the results of our study. No sampling of the SDC was performed to assess the etiology of the collections. In 1 case, there was definite imaging evidence of hemorrhage. In the other cases, no hemorrhage could be documented and the collection contents remain unknown.
Those cases in which sonography provided the only evaluation were excluded, which potentially introduced selection bias. It is possible that those evaluated by cross-sectional imaging may be a different clinical group, with more concern regarding neurologic function, than those evaluated only by sonography. Although sonography can identify large SDC, its sensitivity for smaller collections is limited compared with CT/MRI. We chose not to include this group for the purposes of this study. MRI is the most sensitive test for SDC in this population,21 and small SDC could have been missed in subjects who only had CT imaging. Of the identified SDC in our study, 2 were initially identified by CT, with subsequent confirmation by MRI. The remaining subjects were identified by MRI. A study using only MRI for evaluation would be expected to identify a larger number of SDC. Further prospective studies should consider the use of MRI for evaluation in this cohort. Not all children with SDC received a dedicated clinical assessment for child abuse, limiting the ability to draw definite conclusions on the basis of imaging appearance of the collections. The appearance of some of the collections on imaging (large, complex, and clearly hemorrhagic) probably introduced bias for investigation of potential AHT in these subjects. It is possible that some of the other children with SDC identified in this study were, in fact, victims of child abuse. On the basis of clinical and imaging follow-up available, we believe that this is unlikely.
Conclusions
In summary, our CT and MR imaging study of children with macrocrania demonstrates a 5.6% prevalence of unexpected SDC in children with enlarged SS. No SDC were found in subjects with normal SS. This study, in addition to prior anecdotal and descriptive studies, supports that enlarged SS may predispose these children to small, nonovertly hemorrhagic SDC overlying the convexities, with little or no recognized trauma. Despite these findings, we strongly believe that every unexpected subdural collection in a young child requires close clinical evaluation for underlying causes including AHT. Future studies building on this detailed retrospective investigation are needed to further understand the clinical and pathophysiologic relationships in this scenario.
Acknowledgment
We thank Stephen Boos, MD, for assistance with study design.
Footnotes
Disclosures: Mary V. Greiner—UNRELATED: Expert Testimony: I am a child abuse pediatrician, so I frequently testify as an expert in child abuse in court. I have not testified in any medical malpractice cases; Grants/Grants Pending: 2011: Primary Investigator: A Medical Home for Foster Care Children, The American Academy of Pediatrics Community Access To Child Health (CATCH) Planning Funds, Total awarded: $12,000*; Comments: None of these grants support my salary; Travel/Accommodations/Meeting Expenses Unrelated to Activities Listed: By my institution, Cincinnati Children's Hospital Medical Center, to attend meetings. James Leach—OTHER RELATIONSHIPS: CCHMC has a clinical cooperation agreement with BrainLab AG. I am the physician designate on the agreement (*money paid to institution).
REFERENCES
- Received October 31, 2012.
- Accepted after revision February 27, 2013.
- © 2013 by American Journal of Neuroradiology