Abstract
SUMMARY: Chronic low back and neck pain remain prevalent medical concerns, with much debate regarding the effective evaluation and treatment. Facet disease has been implicated as a source of axial nonradiating low back pain. We discuss patient evaluation, the role of imaging, current and emerging image-guided therapies for facet-related pain, and the increasing importance of outcome-related research in this arena.
ABBREVIATIONS:
- RFA
- radio-frequency ablation
It has been long understood that facet joint degeneration can result from abnormal motion associated with disk degeneration1,2 as well as arthritis, similar to that seen in other synovial joints.3 Proposed pain mechanisms include capsular stretch, entrapment of synovial villi between the articular surfaces, nerve impingement by osteophytes, and release of inflammatory factors.2,4,5 The facet joints have been implicated in 15%–45% of cases of axial low back pain6⇓–8 and 40%–55% of cases of chronic neck pain, without disk herniation.9,10
Early investigations demonstrated that pain could be generated or reproduced by injection of hypertonic saline11,12 into the facet joints, and maps of the pain distribution illustrated the overlap among several dermatome levels. This finding corresponds to the known anatomy, as each facet joint is innervated by small medial branches of the primary ramus from the dorsal root ganglion. Each joint receives innervation from at least 2 spinal levels, including the branch at the level of the named facet joint and a portion from the adjacent level.13 When the facets become pain generators, it is unusual that a single joint is involved. Bilateral involvement has been reported in ≤70% of cases and includes ≥3 regional joints in many patients.14⇓–16
There has been increasing scrutiny during the past 10 years regarding the efficacy and cost of chronic low back and neck pain evaluation and treatment, given their prevalence in society and the impact on the health care system.17 The increasing prevalence of pain relief procedures performed by anesthesiologists, pain management specialists, radiologists, and spine surgeons, among others,18 has drawn the attention of regulatory agencies and societies, resulting in a growing body of evidence-based reviews and practice guidelines.19⇓⇓⇓⇓⇓⇓–26 Controversy persists regarding intervention type, frequency, medication used, and total number of injections. These reviews, however, have served to confirm that there is an increasing demand for more controlled trials and outcome data for these patients and procedures, to justify imaging and image-guided interventions.
Patient Evaluation
Radiologists are often confronted with a nonspecific or incomplete history when interpreting radiographs, CT scans, and MR images of patients with low back pain or neck pain. Facet syndrome has been characterized as low back pain with unilateral radiation to the buttock and/or posterolateral thigh, which may be exacerbated in extension and relieved with flexion. Facet joint pain is often worse after periods of immobilization and improves with motion. Hyperextension and the tilt test can elicit symptoms on examination. Direct pressure over the facet joint during fluoroscopic correlation can also be helpful for isolating symptomatic levels.
Radiographically, the facet joints are best evaluated on oblique projections to avoid superimposed structures.27 CT is more sensitive for evaluation and may reveal degenerative abnormalities such as osteophyte formation, hypertrophy of articular processes, articular cartilage thinning, vacuum joint phenomenon, or calcification of the joint capsule.28 Osteophyte production, joint space narrowing, and fluid within the facet joint can also be evaluated on MR imaging.29⇓–31 Degenerative changes increase with age,32,33 and the above findings are less common in adults younger than 45 years of age.29,34 Many grading systems have been proposed27,29,35,36; however, the severity of imaging findings have not been shown to be specific or to correlate with the severity of symptoms,37,38 and the presence of any imaging abnormality should be correlated with the patient's examination and symptoms.
While imaging findings are not sensitive or specific, imaging does serve an important role in the evaluation of nonradicular spinal pain to exclude confounding processes such as infection, neoplasm, compression fracture, disk disease, or an associated entity such as a synovial cyst, which would require an alternative therapy.
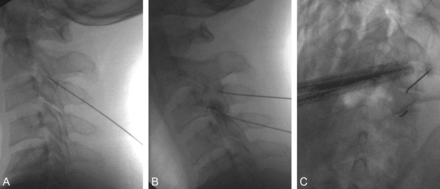
Diagnostic image-guided medial branch nerve blocks have the most convincing evidence (level I) for isolating the facet joint as a pain generator, despite ongoing debate regarding the need for staged serial blocks or placebo-controlled blocks before proceeding to interventional therapy.39 To perform the diagnostic block, the patient should first be examined to establish a baseline pain level. The facet joint is identified fluoroscopically with a C-arm system, and the overlying skin is marked, prepped, and draped in usual sterile fashion. Lidocaine is used to anesthetize the skin and subcutaneous tissues. A 22-ga spinal needle is then inserted percutaneously and advanced under fluoroscopic guidance by using dorsal, lateral, and oblique projections to the junction of the superior articular process and the transverse process for the lumbar spine. For the cervical spine, the anatomic target is the midpoint of the lateral margin of the facet (Fig 1). The exception would be when targeting the third occipital nerve, which is situated along the inferior margin of the C2 facet, adjacent to the C2–3 facet joint. Aspiration should be performed, with no return, before injection of a small volume (0.2 mL) of 2% lidocaine. The patient is then re-examined after a 20-minute interval to assess response to the block.
The left C2–3, C3–4, C4–5, and C5–6 facet joints are fluoroscopically identified in orthogonal planes, with the patient prone, for diagnostic medial branch nerve blocks. The patient had prior cervical disk replacements. Frontal projection (A) confirms proper needle placement at the lateral aspect of the facet joints, and the lateral projection (B) confirms the appropriate depth.
The response to medial branch blocks has been reported to correlate with treatment outcome, despite confounding factors such as psychological comorbidities40; however, given the rate of reported false-positive blocks, placebo controls41,42 have been advocated before progressing to rhizotomy.
Image-Guided Therapy
Facet joint interventions may be considered in patients with >3 months of persistent nonradicular axial spine pain or cervicogenic headache, resulting in functional disability and not responding to conservative medical management or physical therapy.39
Intra-Articular Facet Joint Injection
Mooney and Robertson12 first described facet injections of a mix of steroid and anesthetic in 1976 as a pain relief technique. Case series since that time span the range of results, with good response to injections of steroids43⇓⇓–46 or hyaluronic acid47 for lumbar facet disease, as well as no significant response compared with placebo sham injections.48,49 A paucity of literature reporting outcomes from intra-articular cervical facet injections limits conclusions regarding their broad-based effectiveness.50
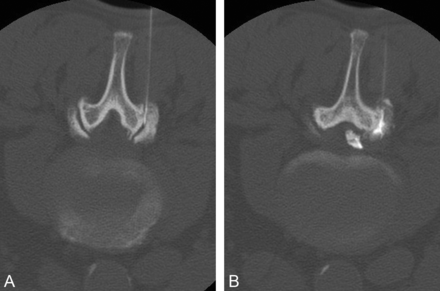
Access to the facet joint can be performed with fluoroscopic (Fig 2) or CT guidance (Fig 3). Fluoroscopy affords the benefit of real-time feedback and multiplanar correlation and, in experienced hands, can minimize radiation dose to the patient. CT guidance requires access to the scanner; however, it can be instrumental for success in accessing joints with steep angles or large overhanging osteophytes. Once the target is identified with the appropriate imaging technique, the overlying skin is marked, prepped, and draped in a sterile fashion, and the skin and subcutaneous tissues are anesthetized. Image guidance is then used to advance a 3.5-inch 22-ga spinal needle to the facet joint, and a small volume (0.2 mL) of contrast is used to confirm the position. We use iohexol (Omnipaque; GE Healthcare, Princeton, New Jersey) as a safety precaution, given its compatibility with intrathecal use. Once intra-articular access is confirmed, a combined solution of anesthetic and steroid can be injected. The most common long-acting steroids include methylprednisolone, triamcinolone, and betamethasone. As with all steroid injections, attention should be given to the total patient steroid dose during a 12-month period, especially in patients with insulin-dependent diabetes.
Fluoroscopic guidance is used for cervical facet intra-articular injection in a patient with chronic nonradicular neck pain and C2–5 facet joint tenderness. The pain was refractory to medial branch nerve ablation 9 months prior. The right C2–3 and C-4 facet joints are identified, and 3.6-inch 22-ga spinal needles are advanced with fluoroscopic guidance in lateral (A), dorsal (B), and oblique projections. C, A test injection of contrast confirms the appropriate placement and excludes vascular access, before injection of steroid and anesthetic.
A, A 3.5-inch 22-ga spinal needle is advanced into the right L4–5 facet under intermittent CT guidance, with the patient prone. B, Injection of 0.2 mL of contrast confirms placement in the joint, and epidural extension is seen after continuing contrast injection. Note that the images are displayed with the patient's right side on the right side of the image, rather than by radiologic convention. We find this orientation easier when performing procedures, especially in the situation of planned bilateral access.
Recent reviews of available literature have concluded that facet joint steroid injections have limited (level III) evidence of benefit,51 are ineffective, or have no benefit.17 A Multisociety Task Force comprising representation from the North American Spine Society, the International Spine Intervention Society, the American Society of Anesthesiologists, the American Academy of Pain Medicine, the American Academy of Physical Medicine and Rehabilitation, the Society of Interventional Radiology, the American Association of Neurologic Surgeons, the Congress of Neurologic Surgeons, the American College of Radiology, the American Society of Spine Radiology, the American Society of Neuroradiology, and the American Academy of Orthopedic Surgeons concluded that 1 therapeutic facet joint injection, per level affected, per year is reasonable if the patient has >50% sustained relief for >3 months and RFA is contraindicated or refused by the patient.52 Intra-articular facet steroid injections may also be considered in patients with prior posterior fusion, in whom access to both medial branch nerves that supply a given level is limited by hardware or bone graft material.
Medial Branch Nerve Ablation
Given at least 50% pain relief in response to medial branch anesthetic blocks, RFA of the medial branch nerve may be considered. A Cochrane Review in 200353 found only 3 high-quality randomized controlled trials evaluating RFA of medial branch nerves as therapy for lumbar facetogenic pain.54⇓–56 Evaluation and conclusions were limited due to the small overall number of patients treated and variations in the diagnostic block method and assessment, outcome assessment, and follow-up duration. Two studies that used comparative placebo blocks demonstrated positive response (>50% relief) at 4–8 weeks54,55 based on functional and pain perception scales, while 156 found no difference in outcomes of the treatment-versus-placebo group at 3 months. Fewer data exist for RFA as a treatment for cervical facet pain. Lord et al41 demonstrated the efficacy of RFA in a double-blind placebo-controlled trial in patients with whiplash injury after motor vehicle crashes, with success defined as a prolonged time of return of pain (median, 8 months) compared with a placebo.
Since then, additional small randomized trials of patients with chronic low back pain who responded positively to diagnostic blocks have been performed. van Wijk et al57 conducted a trial of 81 patients, randomized to radio-frequency treatment or placebo arms. Outcomes assessed at 3 months showed no difference between the groups, though 27%–29% of patients in each group reported improvement in perceived pain during the observation time. Nath et al58 required a response to 3 separate diagnostic blocks as an inclusion criterion. Of the 20 patients treated with multiple radio-frequency lesions at each symptomatic level, 6-month pain-perception scores and range of motion were significantly improved compared with those in the placebo group. A multicenter trial performed for cost-effectiveness randomized patients directly to RFA based on examinations or after a positive response to 1 or 2 comparative diagnostic blocks.59 Of the 84 patients treated, a higher percentage of success was found in the group that did not undergo diagnostic blocks. Overall outcomes showed a 64% positive response at 1 month and 43% at 3 months, with >50% relief considered as success. Gofeld et al60 conducted a 10-year prospective audit including 174 patients who had responded to comparative diagnostic blocks. One hundred nineteen patients (68%) had good (>50%) to excellent (>80%) pain relief lasting 6–24 months; 55 (32%) experienced no benefit from the procedure.
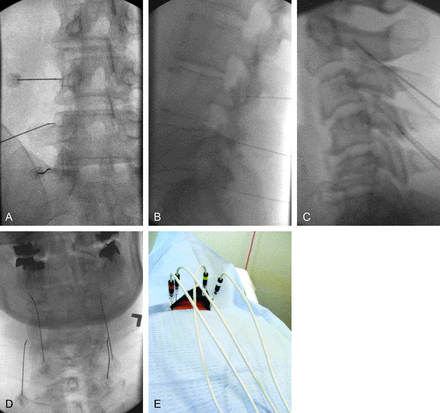
For the procedure, the patient is placed prone and the appropriate levels are identified fluoroscopically. The overlying skin is marked, prepped, and draped in the usual sterile fashion. Lidocaine is used for local anesthesia of the skin and soft tissues, and patients are offered moderate sedation and are monitored by nursing throughout the procedure. At each level, a 22-ga 5- to 15-cm insulated 5- to 10-mm active-tip radio-frequency cannula is inserted percutaneously and advanced under fluoroscopic guidance by using dorsal, lateral, and oblique projections to the base of the superior articular process, the location of the respective medial branch nerves between the intervertebral foramen and the mamilloaccessory ligament at the lumbar levels. Cervical-level targeting is identical to that in the medial nerve block procedure previously described. Aspiration is performed to exclude return of blood or CSF or inducible paresthesia. Needle placement is also confirmed with motor and/or sensory stimulation.
The needles are then injected using a mixture of preservative-free 2% lidocaine and steroid at each level to provide local analgesia during the heating process. Some operators favor dexamethasone in the cervical and thoracic regions, to minimize any risk of particulate-related embolism. The radio-frequency probes are then inserted through the needles and heated in serial fashion either in the radio-frequency mode (80°C for 1.5 minutes) or pulsed mode at (42°C for 2 minutes) (Fig 4). After the heating cycle has finished, the needles are removed and sterile bandages are applied. Postprocedural examination and postsedation monitoring are performed and documented. Complications are rare and include bleeding, infection, or incomplete pain relief.
Frontal (A) and lateral (B) fluoroscopic projections of the lumbar spine with the patient prone confirm appropriate cannula placement for left L2–3, L3–4, and L4–5 medial branch nerve RFA, with the tips of the cannulae at the base of the superior articular process for each level. Frontal (C) and lateral (D) fluoroscopic projections of the cervical spine confirm appropriate cannula placement for bilateral C2–3 and C4–5 medial branch nerve RFA. E, Motor and/or sensory stimulation through each probe is performed in a series, to confirm proximity to the medial branch nerve at each level, before heating.
The suggested retreatment interval is 6–12 months or longer as tolerated, with reports of relief continuing after serial treatments.61 Accelerated repeat RFA treatments may be considered in the setting of recurrent injury or cervicogenic headache.62
With increasing attention on outcomes and the cost-effectiveness of therapy, discussion continues regarding how to best identify patients who will benefit and whether the cost and time required for multiple and/or placebo-controlled diagnostic blocks translates into better patient selection or may exclude patients from receiving beneficial therapy.59 Additionally, debate continues over what constitutes success: Is it 50%, 80%, or 100% relief,63 and what duration? These studies have demonstrated the importance of clearly documenting pretreatment and posttreatment pain perception, functional assessment, and analgesic/opiate requirements.
CT-Guided Decompression of Synovial Cysts
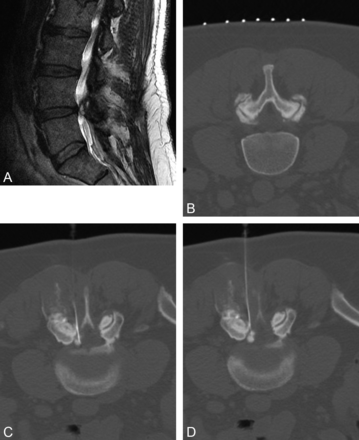
Synovial cysts have been associated with radicular and neuroclaudication symptoms, based on their location, size, and compression of adjacent structures. They also can be an incidental asymptomatic finding on lumbar MR imaging. Because the cyst communicates with the affected facet synovial joint, these lesions can be easily accessed percutaneously with imaging guidance of the needle into the facet joint, though direct puncture of cysts via a sublaminar approach can also be performed (Fig 5). Given the concomitant osteoarthritic changes, CT guidance is often preferred over fluoroscopy to access the joint.
A, Sagittal T2-weighted MR image reveals a large left L4–5 synovial cyst causing moderate central canal stenosis and compression of the exiting left L4 nerve root, corresponding to the patient's radicular symptoms and neuroclaudication episodes. B, CT is used to localize the facet and reveals large overhanging osteophytes limiting joint access. C, CT guidance is then used for a left sublaminar approach. D, Rapid forceful injection of contrast into the cyst is performed; epidural spread of contrast confirms cyst rupture. The patient's pain scale was 8/10 preprocedure and 2/10 postprocedure.
When positioning the patient on the scanner table, it is often helpful to place pillows under the lower abdomen and pelvis, to increase the angle of the target joints rather than relying on angling the gantry alone. Access to the joint is obtained in the same manner as that in an intra-articular injection. A mixture of steroid, anesthetic, and contrast is injected into the joint space, with the goal of rapid expansion resulting in cyst rupture. Success is confirmed by epidural spread of contrast (Fig 6). Accordingly, the anesthetic and steroid in the injectant may help reduce periprocedural inflammation. Serial reports have confirmed the efficacy of this therapy, which may delay or obviate surgical resection of the cysts.64⇓⇓–67 The patient's pain level should be re-assessed within 20 minutes of completing the procedure, to confirm relief from the intervention.
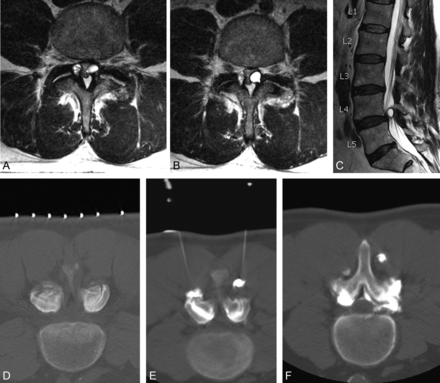
Axial (A and B) and sagittal (C) T2-weighted MR images reveal synovial cysts at L4–5 bilaterally, causing severe central canal stenosis in a patient with neuroclaudication symptoms. A small tail is seen extending from the posterolateral aspect of the left synovial cyst to the left L4–5 facet joint (B), with associated degenerative changes of the facet joint. D, Two 22-ga 3.5-inch spinal needles are advanced with CT guidance into the posterior aspect of the L4–5 facet joints bilaterally, and a test injection of contrast confirms appropriate access to each joint, respectively. E, After forceful injection of contrast mixed with steroid, epidural extension of contrast material is seen, confirming cyst rupture. Contrast extravasation is also noted along the needle trajectory, (F).
Emerging Percutaneous Image-Guided Interventions
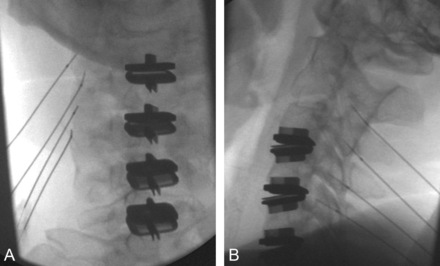
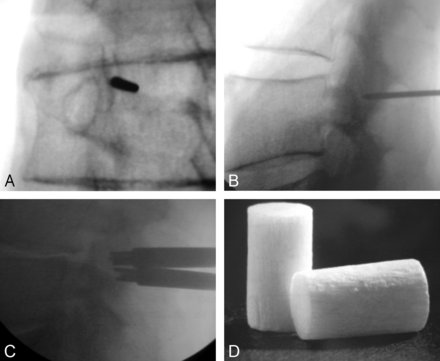
Open pedicle screw fixation has been a common adjunct stabilizing measure with surgical intervertebral body fusion procedures; however, it requires muscle dissection, with related postprocedure morbidity and chronic scarring. Accordingly, percutaneous or minimally invasive methods for transfacet fixation have been desired, and biomechanical and cadaver models have been performed for proof of concept.68⇓⇓–71 Percutaneous innovations for facet screw fixation with hardware72,73 or bone allograft (Fig 7)74 have now reached the market, and technical notes regarding CT75 and fluoroscopic72,76 guidance have been reported in small series. Concerns have been expressed about the use of facet fixation as an independent procedure in the outpatient setting, rather than as an adjunct to lumbar interbody fixation, as well as about complications from bone allograft dowel migration. Insufficient independent data are available to assess the safety, efficacy, or outcomes of these procedures.77
Frontal (A) and lateral (B) projections confirm the appropriate trajectory for the guide pin extending to the facet joint. A small incision is then made in the skin, and the spatula and drill devices are sequentially advanced into the facet joint to form the appropriate cavity. The tamping device is then advanced (C) to seat the dowel of bone allograft material (D). Note that the allograft is difficult to see under fluoroscopic guidance (C). Image D provided courtesy of Trufuse (Clearwater, Florida).
Conclusions
Neuroradiologists need to be aware of the role of facet joints as a possible independent or contributory pain generator in the evaluation of patients with chronic low back pain. While imaging findings are not specific to implicate the facet joints, imaging is extremely useful to exclude nondegenerative pain etiologies as well as to identify confounding entities such as synovial cysts, which would have treatment implications. Fluoroscopically guided medial branch nerve blocks are emerging as a standard of care to confirm the facet joint as a pain generator and to isolate the appropriate levels. These can be followed by thermal ablation of the medial branch nerve under fluoroscopic guidance; however, additional scrutiny of these procedures, the numbers of levels treated in a single visit, and the frequency of re-treatment remain areas of continued debate in the literature, with emphasis on outcomes research. Additional image-guided minimally invasive therapies such as percutaneous facet fixation are emerging, though they have not been performed in sufficient numbers to understand long-term efficacy or complications. Neuroradiologists interested in treating patients with chronic facet degenerative pain should be aware of the options and the role of physical examination and history in addition to imaging, as well as the need to closely track and report outcomes.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- © 2012 by American Journal of Neuroradiology