Abstract
BACKGROUND AND PURPOSE: Oligodendrogliomas are tumors that have variable WHO grades depending on anaplasia and astrocytic components and their treatment may differ accordingly. Our aim was to retrospectively evaluate imaging features of oligodendrogliomas that predict tumor grade.
MATERIALS AND METHODS: The imaging studies of 75 patients with oligodendrogliomas were retrospectively reviewed and compared with the histologic grade. The presence and degree of enhancement and calcification were evaluated subjectively. rCBV and ADC maps were measured. Logistic linear regression models were used to determine the relationship between imaging factors and tumor grade.
RESULTS: Thirty of 75 (40%) tumors enhanced, including 9 of 46 (19.6%) grade II and 21 of 29 (72.4%) grade III tumors (P < .001). Grade III tumors showed lower ADC values compared with grade II tumors (odds ratio of a tumor being grade III rather than grade II = 0.07; 95% CI, 0.02–0.25; P = .001). An optimal ADC cutoff of 925 10−6 mm2/s was established, which yielded a specificity of 89.1%, sensitivity of 62.1%, and accuracy of 78.7%. There was no statistically significant association between tumor grade and the presence of calcification and perfusion values. Multivariable prediction rules were applied for ADC < 925 10−6 mm2/s, the presence of enhancement, and the presence of calcification. If either ADC < 925 10−6 mm2/s or enhancement was present, it yielded 93.1% sensitivity, 73.9% specificity, and 81.3% accuracy. The most accurate (82.2%) predictive rule was seen when either ADC < 925 10−6 mm2/s or enhancement and calcification were present.
CONCLUSIONS: Models based on contrast enhancement, calcification, and ADC values can assist in predicting the grade of oligodendrogliomas and help direct biopsy sites, raise suspicion of sampling error, and predict prognosis.
ABBREVIATIONS:
- CI
- confidence interval
- rCBV
- relative cerebral blood volume
- ROC
- receiver operating characteristic analysis
- SE
- spin-echo
- WHO
- World Health Organization
Among the gliomas, oligodendrogliomas are the third most common, which account for 5%–20% of all glial tumors.1⇓–3 They are slow-growing tumors, most often arising in the frontal lobe.3⇓–5 More commonly found in the adult population, these tumors usually present in the fifth and sixth decades of life.4,6,7 Clinical presentation is usually seizures and headaches.4,8
The WHO classification divides oligodendrogliomas histopathologically as low-grade (WHO II); high-grade or anaplastic (WHO III); anaplastic oligoastrocytomas with necrosis are classified as glioblastomas (WHO IV).9 Low-grade tumors may progress to high-grade tumors. However, this transformation is difficult to predict.10
MR imaging is the best technique to diagnose these tumors. Studies have looked at MR imaging contrast enhancement,11⇓–13 calcifications,14 and the use of diffusion and perfusion imaging to predict grade and anaplasia.15⇓–17 However, no uniform predictive model presently exists for radiologic determination of the grade of these tumors.18
The purpose of this study was to retrospectively evaluate imaging features of oligodendrogliomas that predict tumor grade. We also sought to assess whether contrast enhancement is seen as often as reported in the oligodendroglioma literature. In a similar fashion, we sought to assess whether tumoral calcification, perfusion values, and ADC values on MR imaging can be used in a predictive model for radiologic assessment of tumor grade. Tumor grade is important for directing biopsy sites, staging accurately, proving prognosis to patients, and raising suspicion of sampling error when discordant pathology and radiology studies are present.
Materials and Methods
After institutional research board approval and with Health Insurance Portability and Accountability Act compliance, 75 patients diagnosed with oligodendroglioma from the Johns Hopkins Hospital data base, who underwent MR imaging from 2004 to 2010, were retrospectively evaluated. The 75 patients had biopsy-confirmed oligodendroglioma, classified by an experienced neuropathologist according to the WHO criteria. Forty-six oligodendrogliomas were low-grade (WHO II), and 29 were high-grade (WHO III). Of the low-grade oligodendrogliomas, 44 were pure oligodendrogliomas and 2 were oligoastrocytomas. Of the high-grade tumors, 28 were anaplastic oligodendrogliomas and 1 was an anaplastic oligoastrocytoma.
MR imaging was performed at either 1.5T or 3T. T1 SE, T2-weighted, and FLAIR fast SE images were acquired. The sagittal T1-weighted images were obtained with parameters as follows: TR range, 9.89–696 ms/TE range, 4.6–14 ms; matrix size from 192 × 192 to 512 × 196; FOV from 190 × 190 mm to 240 × 240 mm; range of section thickness/spacing of 1/1–5/7 mm.
The axial T2-weighted images were obtained with a TR range, 2500–7000 ms/TE range, 83.136–112 ms; matrix size from 256 × 184 to 448 × 335; FOV from 159 × 200 mm to 240 × 240 mm; range of section thickness/spacing of 2/2–5/5.
FLAIR scan parameters were the following: TR = 6000, TE = 120, TI = 2000, section thickness = 5 mm, FOV = 23 cm, and matrix size = 256 × 256.
DWI was performed with the EPI sequence with a TR/TE range, 4900–10000 ms/80–133 ms; 5-mm thin contiguous sections; FOV of 220 × 220 mm to 240 × 240 mm; and a matrix size of 96 × 96 to 192 × 192. Diffusion was measured in the 6 orthogonal directions with 2 b-values (0 and 1000 s/mm2) with automated postprocessed ADC maps. Perfusion images by using a first-pass gadolinium technique were obtained with a 90° flip angle and TR values between 1300 and 2000 ms and TE values between 40 and 60 ms. Ten minutes after injection of gadopentetate dimeglumine (0.1 mmol/kg of body weight) postcontrast T1-weighted SE images were acquired. All sequences had a section thickness of 5 mm and a section gap of 0–2.5 mm.
Two radiologists who were blinded to the histologic grade of the oligodendroglioma reviewed the studies independently. They reached a consensus regarding the presence or absence of tumor contrast enhancement. If they failed to reach consensus, a third neuroradiologist reader provided the deciding value for most opinions. The precontrast and postcontrast images were windowed and leveled identically to minimize any discrepancy in evaluating tumor contrast enhancement. Enhancement was scored as 1 (no enhancement), 2 (minimal enhancement), 3 (moderate enhancement), and 4 (severe enhancement) (Table 1). Disagreements were settled by consensus readings.
Number of cases and their enhancement characteristics
DWI sequences and ADC maps were reviewed, and ADC values were calculated. The most solid and lowest signal-intensity part of the tumor on ADC maps was marked as the ROI, and 4 different values of the intensity were noted within these regions, with the readers blinded to other pulse sequences. The area of lowest signal intensity on the ADC map was used, and there was no attempt to correlate this area with either 1) calcification, 2) enhancement, or 3) perfusion results. Care was taken in ensuring that all 4 ROIs were completely within the tumor in the 4 areas of lowest ADC. Each ROI was between 8 and 10 mm2. The T2-weighted and/or FLAIR images and/or enhanced T1-weighted scans were used to accurately identify tumor location for the ADC maps. The lowest value of the 4 ROIs with its SD was recorded. We did not correlate or collocate the ADCs with the gadolinium enhancement, calcification, or perfusion data. We recorded the lowest values on the basis of visual inspection of ADC map and sampling of 4 ROIs, taking the lowest of these 4 values. These were then averaged for 1 common value between the 2 radiologists. If the value for 1 reader varied by >20% from the second reader, a third reader measured the ADC value, and the average of the 2 closest values (corrected values) was used. Using a third reader was required in 19 (25.3%) of the 75 cases. The mean coefficient of variation (difference between reads divided by the mean of the 2 reads) of the corrected 75 ADC values was 8.4% (SD, 6.5%).
Measurements of rCBV were available for 20 cases. Dynamic susceptibility-weighted contrast-enhanced perfusion images were processed off-line by using in-house software, including a correction for blood-brain barrier permeability.19 Within the ROIs (8–10 cm2), the highest CBV value was noted and averaged. Perfusion values were selected separately and independently from the ROIs of the ADC values (ie, they were not in the same areas necessarily). When marking the ROI, we took great care to avoid regions of necrosis, calcification, cyst, or edema, aiming for the most solid part of the tumor. The final rCBV values used expressed the lesion CBV relative to CBV in the contralateral centrum semiovale white matter. Because this was a retrospective study, no standard protocol was used for imaging the tumors. Consequently, not all subjects included underwent perfusion studies.
The presence and absence of calcification were evaluated qualitatively on CT (n = 45) images. Preoperative CT scans were not available for all patients because of the lack of a standardized imaging protocol and the retrospective nature of the study. The presence of calcification and other imaging findings was compared with the histologic grade of tumor.
Prevalence proportions were estimated by using sample proportions, and corresponding CIs were constructed by using the score interval along with the Yates continuity correction.20,21 Logistic linear regression models were used to determine the relationship between given predictors and the outcome of interest; these provided odds ratios along with CIs and P values obtained by inverting asymptotic Z-tests.20,21 Cross-validation was performed in each computation of the area under the ROC curve. When continuous predictors were dichotomized, the cutoff used was that which resulted in the classification rule with the greatest sum of sensitivity and specificity; this ensured a balanced trade-off between sensitivity and specificity. All computations were implemented by using the R statistical language (R Foundation for Statistical Computing, Vienna, Austria).22
Results
Of the 75 tumors, 30 (40.0%; 95% CI, 29.1%–52.0%) showed contrast enhancement. Enhancement was more common in grade III tumors, being observed in 9 of 46 (19.6%; 95% CI, 9.9%–34.4%) grade II tumors but 21 of 29 (72.4%; 95% CI, 52.5%–86.6%) grade III tumors (Table 2). The odds of a tumor being grade III versus grade II were estimated to be 10.8 times greater when some enhancement was present (enhancement score, ≥2) versus when none was found (enhancement, 1) (odds ratio = 10.8; 95% CI, 3.6–32.2; P < .001) (Fig 1).
Grade II oligodendroglioma. A, Axial T2-weighted fast SE image shows a high-signal-intensity mass in left frontal lobe. B, Axial contrast-enhanced T1-weighted image shows no postcontrast enhancement within the left frontal lobe mass. C, Axial automated postprocessed ADC map yields a minimal ADC value of 946 × 10−6 mm2/s, above the 925 × 10−6 mm2/s threshold.
Contrast enhancement in oligodendrogliomas
Tumors of grade III yielded lower ADC values, with a median ADC value of 1071 × 10−6 mm2/s (first quartile: 1000 × 10−6 mm2/s; third quartile: 1253 × 10−6 mm2/s) in grade II tumors and of 908 × 10−6 mm2/s (first quartile: 835 × 10−6 mm2/s; third quartile: 1021 × 10−6 mm2/s) in grade III tumors (Fig 2). ADC values were found to have relatively good predictive ability, with an area of 0.75 under the ROC curve. The optimal dichotomization cutoff value of ADC for classifying tumors as either grade II or III was identified as 925 × 10−6 mm2/s, with tumors with ADC values ≤ 925 × 10−6 mm2/s being deemed grade III, and those with >925 × 10−6 mm2/s, grade II. This classification rule for identifying grade III tumors resulted in a specificity of 89.1%, sensitivity of 62.1%, negative predictive value of 78.9%, positive predictive value of 78.3%, and accuracy of 78.7%. Furthermore, tumors with a low ADC value (ADC ≤ 925 × 10−6 mm2/s) were estimated to have odds of being of grade III 13.4 times greater than tumors with a high ADC value (ADC >925 × 10−6 mm2/s) (odds ratio = 13.4; 95% CI, 4.1–44.3; P < .001).
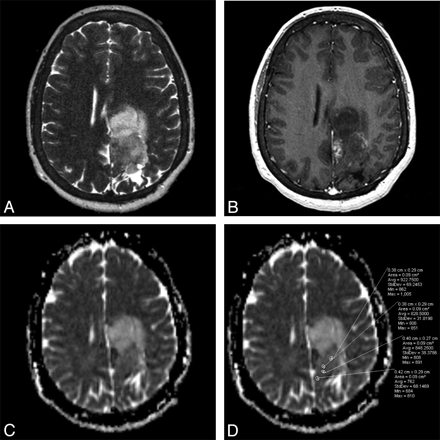
Grade III oligodendroglioma. A, Axial T2-weighted fast SE image shows a high-signal-intensity mass in left medial frontal and parietal lobes. B, Axial contrast-enhanced T1-weighted image shows minimal nodular post–contrast enhancement within the medial margin of the mass. C, Axial automated postprocessed ADC map shows low signal intensity along the posterior margin. D, As stated in “Materials and Methods,” 4 ROIs are placed in the areas visually assessed as the lowest in signal intensity on the ADC map. In this case, of the 4 ROIs, the lowest ADC value is 762 × 10−6 mm2/s, which was recorded for this grade III oligodendroglioma, below the 925 × 10−6 mm2/s threshold.
Calcification data were available for 45 of 75 cases. Calcification was more common in grade III tumors, being identified in 6 of 24 grade II tumors (25.0%; 95% CI, 10.6%–47.1%) and in 11 of 21 grade III tumors (52.4%; 95% CI, 30.3%–73.6%), though this difference was not statistically significant. The odds of a tumor being of grade III versus grade II were 3.3 times higher in the presence of calcification compared with absent calcification (odds ratio = 3.3; 95% CI, 0.9–11.6; P = .063). As a classification rule for establishing tumor grade, with noncalcified and calcified tumors being deemed grades II and III, respectively, calcification had a specificity of 75.0%, sensitivity of 52.4%, negative predictive value of 64.3%, positive predictive value of 64.7%, and accuracy of 64.4%.
Perfusion data were available for 20 of 75 cases. Tumors of grade III were found to exhibit lower perfusion values, with a median perfusion value of 2.12 (first quartile: 1.55; third quartile: 3.23) in grade II tumors and of 1.67 (first quartile: 1.39; third quartile: 21) in grade III tumors. The difference in perfusion values between groups was not statistically significant.
Classification rules involving several of the predictors discussed above were studied. Because of the large number of missing values, its lack of a significant association with tumor grade, and its overall poor value as a predictor, perfusion was excluded from multivariable analyses. The remaining 3 predictors (ADC value, enhancement, and calcification) jointly yielded an ROC curve with area of 0.88, indicating that together they enjoyed very good predictive ability. The 17 multivariable prediction rules constructed by using the dichotomizations of ADC values, enhancement, and calcification were evaluated. These rules are provided in Table 3 along with their characteristics. Symbols A, E, and C refer to an ADC value ≤925 × 10−6 mm2/s, the presence of enhancement, and that of calcification, respectively. As such, the rule A + C corresponds to predicting a tumor to be grade III if both an ADC value ≤925 × 10−6 mm2/s and calcification were observed, for example, whereas the rule E + (A or C) corresponds to predicting grade III if both enhancement is observed and either the ADC value is ≤925 × 10−6 mm2/s or calcification is present.
Performance of 17 multivariate prediction rulesa
Nine of these rules could be eliminated because each of these was outperformed in all characteristics by at least 1 other rule (Table 3). Of the remaining 8 rules, 3 were identified as having particularly good overall performance, as characterized by accuracy and the sum of sensitivity and specificity. Predicting a tumor to be grade III if either of the ADC value is ≤925 × 10−6 mm2/s or enhancement is observed (A or E in Table 3) resulted in 73.9% specificity, 93.1% sensitivity, 94.4% negative predictive value, 69.2% positive predictive value, and 81.3% accuracy. This rule had the highest sum of sensitivity and specificity. Rendering this rule slightly more stringent by predicting a tumor to be grade III if either the ADC value is ≤925 × 10−6 mm2/s or both enhancement and calcification are observed (A or [E + C] in Table 3) resulted in a more balanced rule, with all measures of performance above 80%. Specifically, this rule had a specificity of 83.3%, a sensitivity of 81.0%, a negative predictive value of 83.3%, a positive predictive value of 81.0%, and an accuracy of 82.2%, the highest accuracy of all rules investigated.
Discussion
Grading oligodendrogliomas accurately is important because it has significant implications for the choice of technique used for the management of these tumors. Treatment for low-grade gliomas is primarily surgical resection and radiation therapy.13 Anaplastic oligodendrogliomas, on the other hand, show a favorable response to procarbazine, vincristine, lomustine, and temozolomide.23 Sampling errors and inconsistency in histopathologic grading of tumors can lead to over- or undertreatment of these tumors. Our predictive model tries to limit these errors in clinical management by providing a noninvasive tool with good predictive power to assess the grade of tumor.
Oligodendrogliomas have been reported to show frequent postcontrast enhancement on MR imaging.12 Gadolinium enhancement is suggested to be proportional to tumor vascularity.24 Most studies report that 50%–60% of oligodendrogliomas show contrast enhancement and that this feature is associated with high-grade tumors.11,24,25 Our results suggest that rate of enhancement of oligodendrogliomas is 40% (30/75). This is less than the reported 60% enhancement based on analysis of the literature. Among the enhancing tumors, a greater number of grade III tumors showed enhancement as opposed to grade II tumors, and this difference in enhancement is statistically significant. This result is different from the finding reported by White et al,25 who reported no difference in enhancement with grades of tumor. This significant difference in enhancement rates can help in differentiating low-grade and high-grade tumors. As an independent predictor of tumor grade, we found it not to be very specific. A reason could be other factors influencing contrast enhancement rather than just the degree of neovascularity of the tumor, which correlates to the aggressiveness of tumor. Some studies have proposed a disruption of the blood-brain barrier as a contributing cause.25
ADC values have shown high predictive power when it comes to differentiating high-grade and low-grade astrocytomas. Previous studies have used ADC maps on DWI to determine the tumor grade.15,26 An association between tumor cellularity and ADC has been proposed in the literature.26 High-grade tumors are reported to have low ADC values, likely due to water restriction within these tumors secondary to a high nucleus-to-cytoplasm ratio and hypercellularity and higher ADC values in low-grade tumors.27 Our results support this by demonstrating a statistical difference between ADC values for grade III and grade II tumors. To establish the ADC value as a strong predictor, we propose a threshold value of 925 × 10−6 mm2/s because this had the highest sum of sensitivity and specificity. ADC values may, therefore, be useful in assessing the grade of tumor, and ADC maps may also allow better localization of biopsy sites.
Calcification is a common finding in oligodendrogliomas.3 Calcifications were seen more frequently in grade III tumors rather than grade II tumors, though this was not statistically significant. A reason for this could be the limited sample size, and this can be overcome in future studies by increasing the number of cases. We found that calcification increases the power of our predictive model when used in conjunction with ADC and enhancement. Also the presence of calcification in an enhancing tumor is highly suggestive of a grade III oligodendroglioma. This will go on to increase the diagnostic confidence and help in guiding clinical management and support biopsy results.
Dynamic susceptibility contrast MR imaging data can be analyzed to generate various perfusion parameters in the brain, such as mean transit time and relative CBF and rCBV. For the evaluation of tumors, the most commonly used parameter is rCBV, which is believed to reflect lesion vascular proliferation and attenuation.28 rCBV is often reported to be elevated in glial brain tumors compared with normal white matter, with rCBV values increasing with tumor grade, particularly for astroglial tumors.15,29 While some investigators have found increasing CBV with increasing grade in oligodendroglioma,30,31 others have found this not to be the case,32,33 with high rCBV often found in low-grade oligodendrogliomas, as was also observed in the current study. However, the rCBV results in the current study may not be particularly representative (and lack statistical significance) at least, in part, because of the relatively small number of subjects who underwent MR perfusion imaging in this retrospective study (20 of 75). It is possible that a larger study may find significant rCBV associations with grade in oligodendroglioma.
rCBV measurements in oligodendroglioma may still be clinically useful (eg, in terms of predicting treatment response or disease progression) even if they do not correlate with tumor grade. For instance, Whitmore et al30 have found increased rCBV in low-grade oligodendroglioma with 1p/19q loss of heterozygosity; oligodendrogliomas with these mutations generally show a good response to chemotherapy. Also, another study has found strong negative correlations between CBV and time to progression in patients with low-grade gliomas (both astrocytomas and oligodendrogliomas), with low CBV indicating longer time to disease progression.34
As shown by multivariate association when the ADC threshold, enhancement, and calcification are used in conjunction, the predictive power of each measure increases significantly. Our predictive model based on the ADC value by using a cutoff of 925 × 10−6 mm2/s, the presence or absence of contrast enhancement, and calcification provides a promising noninvasive tool in guiding both surgical and clinical management of oligodendrogliomas. Using this model can guide physicians to accurate biopsy sites, reduce sampling error, and allow earlier decisions regarding management. The predictive models presented in this study are not powerful enough to replace histopathologic evaluation, which should still remain the criterion standard for diagnosing grade of tumor. The predictive models provide the advantage of time because they are easily obtainable and can influence decision-making while the tissue analysis results are pending.
The predictive power of our model can be improved by using a larger sample size in prospective studies with standardized protocols on magnets of similar field strength and the same manufacturer, for MR imaging of oligodendrogliomas. Because enhancement was judged subjectively, there is always a chance that subtle enhancement could have been missed. We tried to overcome this by minimizing interobserver variability and having a third radiologist act as the tiebreaker. In future studies, a more quantitative method to grade enhancement can be used to overcome this problem. Another limitation could be the placement of ROIs for measurement of ADC and CBV values, which was also performed subjectively because tumor borders are ill-defined. Although care was taken to place the ROIs entirely within the tumor, some variations in perfusion and ADC values could be secondary to inclusion of nontumorous areas. We tried to overcome this limitation by placing 4–5 ROIs within the tumor and averaging these to decrease the chance of an incorrect measurement. On the basis of incomplete 20-subject data, we cannot confidently rule out perfusion as a predictor of tumor grade. In future studies, more cases should be used to validate the predictive model, and a relationship between the predicted grade of tumor and survival rate can help establish the significance of these predictive parameters on MR imaging.
Conclusions
MR imaging contrast enhancement in oligodendrogliomas in the current series was less prevalent (40%) than reported in the prior literature (60%). Enhancement when used with the presence of calcification and/or an ADC threshold value of 925 × 10−6 mm2/s can act as an accurate noninvasive predictor of tumor grade. This predictive model provides a reliable and rapid tool to better guide physicians when it comes to the localization of biopsy sites and can also facilitate earlier decision-making and management. Histopathologic analysis of the tumor should still be considered the criterion standard for establishing tumor grade.
Acknowledgments
We acknowledge the effort and contributions of Gloria Vila and John Boitnott for providing us with the list of cases for our study.
Footnotes
Disclosures: Netsiri Dumrongpisutikul—UNRELATED: Employment: King Chulalongkorn Memorial Hospital, Thai Red Cross Society, Grants/Grants Pending: King Chulalongkorn Memorial Hospital, Thai Red Cross Society. Peter Barker—UNRELATED: Grants/Grants Pending: Philips Healthcare.
References
- Received June 22, 2011.
- Accepted after revision September 4, 2011.
- © 2012 by American Journal of Neuroradiology