Abstract
SUMMARY: High-resolution MRN is becoming increasingly available due to recent technical advancements, including higher magnetic field strengths (eg, 3T), 3D image acquisition, evolution of novel fat-suppression methods, and improved coil design. This review describes the MRN techniques for obtaining high-quality images of the peripheral nerves and their small branches and imaging findings in normal as well as injured nerves with relevant intraoperative correlations. Various microsurgical techniques in peripheral nerves, such as neurolysis, nerve repairs by using nerve grafts, and conduits are discussed, and MRN findings of surgically treated nerves are demonstrated.
ABBREVIATIONS:
- AP
- anteroposterior
- CPN
- common peroneal nerve
- IV
- intravenous
- LPN
- lateral plantar nerve
- LR
- left-to-right
- MIP
- maximum intensity projection
- MPN
- medial plantar nerve
- MPR
- multiplanar reconstruction
- MRN
- MR neurography
- SI
- signal intensity
- SNR
- signal-to-noise ratio
- SPACE
- sampling perfection with application-optimized contrasts by using different flip angle evolutions
- SPAIR
- spectral-attenuated inversion recovery
- STIR
- short tau inversion recovery
- T1WI
- T1-weighted imaging
- T2WI
- T2-weighted imaging
- TSE
- turbo spin-echo
Better understanding of the neurobiology and neuroimmunology of nerve regeneration has led to implementation of new and refined techniques in peripheral nerve surgery, resulting in dramatically improved functional outcomes.1–3 More than 100,000 peripheral nerve surgery procedures have been performed in the United States and Europe annually since the early 1990s.4 The ability of MR imaging to demonstrate nerve changes in response to injury has been scientifically investigated in multiple small-animal models.5–7 While conventional MR imaging may show the indirect signs of nerve damage such as muscle denervation and edema,8,9 high-resolution MRN allows direct visualization of the injured and entrapped nerves, including their smaller peripheral branches. Various technical advancements, including higher field strengths (eg, 3T), 3D image acquisition, evolution of fat-suppression methods, and improved coil design allow increasingly high-quality MRN images.10,11 Noninvasive assessment of peripheral nerve injury by MRN aids in improved diagnosis and presurgical planning.12 Additionally, MRN may also provide evidence of nerve healing or degeneration.13 MRN can be harnessed as a powerful tool, when applied to an appropriate clinical question supplemented by information obtained from the patient history, clinical examination, and electrodiagnostic studies, such as electromyography and quantitative neurosensory testing.13–18 This review highlights the role of high-resolution MRN in demonstrating normal peripheral nerves, their preoperative pathologic appearances, and postsurgical findings.
MRN Technique
Apart from T2-based techniques, which reliably enable high-resolution and high-contrast imaging of peripheral nerves, diffusion-based MRN has also been studied.10,19 However, outside the central nervous system, diffusion-weighted imaging is relatively experimental and can be technically challenging to perform and interpret. T2-based techniques offer multiple advantages such as ease of protocol implementation, reproducibility of imaging, proved validity, and familiarity to radiologists.20–22 A combination of high-resolution axial T1WI (for anatomy) and high-resolution and high-contrast axial fat-suppressed T2WI (for pathology) is essential for detailed evaluation of peripheral nerves (Fig 1).23–25 Due to abundant perineural and intraneural fat interspersed among the individual fascicles, these images are helpful in distinguishing the fascicular pattern of the nerve from adjacent vessels.15 Fat-suppression sequences are most useful for assessment of abnormal nerves due to pathologically increased endoneurial fluid, which could indicate nerve injury or entrapment. There are multiple ways to suppress background fat to enhance the relative nerve SI and contrast. STIR generally works best at 1.5T and provides homogeneous fat suppression. Chemical fat saturation is also an option but may produce nonuniform fat suppression, limiting the evaluation. SPAIR sequences usually provide adequate and uniform fat suppression and better SNR compared with STIR sequences (Fig 1B).13,26 3D sequences such as 3D T2-weighted SPACE (Fig 2) and 3D STIR SPACE provide isotropic voxel resolution with spin-echo contrast on 3T scanners within acceptable imaging times.27 Isotropic 3D acquisition allows arbitrary MPR and MIP at postprocessing, which can be helpful in demonstrating subtle nerve contour or course abnormalities. In the postoperative setting, another 3D sequence, fat-saturated T1 volumetric interpolated breath-hold examination, is used to detect postoperative complications, such as abscess, hematoma, neuritis, and so forth.26 Due to their close proximity, distinguishing between smaller nerve branches and their accompanying vessels is often aided by administration of IV contrast (gadolinium). Saturation bands may also be useful to suppress vascular SI within the neurovascular bundle. However, small vessels, especially slow-flowing veins, may not become saturated; the result is suboptimal small peripheral nerve imaging. Therefore, we do not routinely using saturation bands.
MRN appearance of a normal CPN. Axial T1-weighted (A) and axial T2 SPAIR (B) images at the level of popliteal fossa show an example of a normal CPN (straight arrows). Note the fascicular appearance and isointensity of the CPN on T1WI and T2WI with respect to the skeletal muscle. The nerve also appears normal in size and is surrounded by a rim of perineural fat (curved arrow) and thin epineurium.
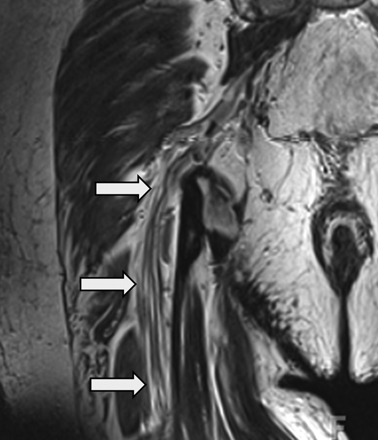
MRN appearance of a normal sciatic nerve. Coronal T2 SPACE image demonstrates a normal sciatic nerve (arrows). Individual fascicles outlined by fat are clearly seen coursing in the craniocaudal direction. Note the iso- to minimal T2 hyperintensity of the normal nerve with respect to the skeletal muscle. The nerve also appears normal in size and is surrounded by a rim of perineural fat.
The choice of technique for a particular examination is optimally guided by the clinical question as well as the anatomic area of interest. It is prudent for the radiologist, referring clinicians, and MR imaging technologists to work in close collaboration for formulation and selection of protocols. An approach of a larger FOV to exclude proximal pathology and target zone (10–15 cm) imaging at the area of interest are routinely used. The Table shows a generic high-resolution MRN protocol.
Typical protocol for 3T MR imaging sequences used for MRN of the sciatic nervea
Anatomy and Pathophysiology
Detailed knowledge of the nerve structure is essential for understanding the pathology of peripheral nerve injury. A single nerve fiber, the axon, is considered the functional unit of a peripheral nerve. Each axon is surrounded by a connective tissue layer, referred to as the endoneurium. Multiple axons group to form a fascicle. The connective tissue layer that surrounds each fascicle is called the “perineurium.” The perineurium maintains an intrafasciular pressure and provides tensile strength and protection from stretch injury. The final layer, called the “epineurium,” separates the fascicles as well as loosely binds them together.3
Traditionally, nerve injury has been classified into 3 grades by increasing severity. Nerve injury without axonal disruption is termed “neurapraxia”; it is usually transient and self-limiting with complete recovery. Axonal disruption with preservation of the myelin sheath, or “axonotmesis,” is a more severe injury in which the axon is damaged but supporting connective tissue structures of the nerve remain intact. The most severe grade is “neurotmesis,” which is characterized by loss of axonal continuity along with disruption of the surrounding myelin sheath and connective tissue.3,28 In milder degrees of axonotmesis, regeneration may restore full function. Spontaneous recovery in neurotmesis and severe axonotmesis is rare, and these conditions usually require surgical intervention for functional recovery. Axonotmesis may lead to formation of a neuroma-in-continuity, and neurotmesis may lead to formation of an end bulb neuroma.29,30 Injury to the nerve results in a cascade of cellular and molecular responses leading to Wallerian degeneration of the distal nerve segment. Monocytes enter the damaged endoneurial sheaths and clear debris. In a successful regenerative response, eventually transformed monocytes (macrophages) participate with Schwann cells to provide trophic (feeding) and tropic (guidance) factors for regenerating and growing nerve sprouts, axonal elongation, and re-ensheathment.
MRN Findings in Abnormal Peripheral Nerves
MRN findings in injured nerves include a variable combination of focal or diffuse enlargement of the nerve, abnormal hyperintensity of all or some fascicles on T2-weighted images approaching the intensity of adjacent hyperintense venous structures, disruption of a fascicular pattern, displacement or altered course (ie, nerve kinking), formation of a traumatic neuroma, and perineural low SI (best seen on T1-weighted images), suggesting fibrosis (Fig 3 A, -B).13,22,31 Many causes of hyperintensity on T2-weighted images have been hypothesized; however, the complete pathoetiology is unclear. Endoneurial and/or perineurial edema, venous congestion, obstruction of the axoplasmic flow and altered blood nerve barrier, and Wallerian degeneration may all play a role in isolation or combination.32,33 The highest SI and loss of fascicular pattern of injured nerves on T2-weighted images is seen just proximal to and at the site of entrapment.34,35 The abnormal T2 SI gradually fades and resolves distally. However, the reader should be aware of some important pitfalls. Minimal T2 hyperintensity, as an isolated finding, may be normally seen in some nerves, such as the ulnar nerve, the sciatic nerve at the sciatic notch, and the medial planter nerve.36 Additionally, the SI may vary due to magic angle artifacts; therefore, it is recommended that the nerve in question be evaluated in multiple planes and that higher TE values of >60 ms be used for T2WI to avoid this pitfall.
MRN appearance of abnormal CPN with axonotmesis. Axial T1WI (A) and axial T2 SPAIR (B) images of a 25-year-old man with clinical findings of left CPN related to a previous injury. The rim of perineural fat is disrupted with fibrosis on T1WI (curved arrow). Note the enlarged and T2 hyperintense CPN (white arrows) with lost fascicular appearance and thickened epineurium (a dark SI rim surrounding the hyperintense CPN). Regional denervation muscle edema and atrophy in the muscles of the extensor compartment were also seen (not shown). C, Intraoperative photograph confirms a thickened epineurium surrounding the CPN and perineural fibrosis (arrows).
Nerve Microsurgery
Microsurgical techniques have benefited greatly in last 20 years from the ability to perform surgery under magnification. Fibrotic tissue within and around the partially injured peripheral nerve is removed by neurolysis.37 In cases of severe axonotmesis/neurotmesis, direct nerve repair is attempted. Reconnecting the 2 ends of the injured nerve directly (end-to-end repair, coaptation) may be considered in limited instances because approximation of nerves with minimal tension may be difficult to achieve. Direct repair is also likely to be more successful when the nerves are purely motor or purely sensory and when the amount of intraneural connective tissue is relatively small.38,39 This is technically challenging because nerve stumps must be repaired with minimal tissue damage, using a minimum of sutures or glue and requiring that the stumps be precisely aligned without tension.40,41 The current criterion standard of repair for nerve gaps is autologous nerve grafting.42,43 Nerve grafts, either allo- or autografts, are in use clinically and may be used as end-to-side or end-to-end repairs. The sural nerve (a sensory nerve) is the most commonly used donor nerve for autologous nerve grafting. However, introducing 2 suture lines increases the possibility of complications such as neuroma-in-continuity formation and intraneural fibrosis.44
Conceptually, these complications could be prevented by the use of nerve guides or conduits so that the regenerating nerve fibers are allowed to grow toward the distal nerve stump, while reducing the risk of neuroma formation and in-growth of fibrous tissue into the nerve gap. In the past decade, various studies by using conduits, either of biologic or synthetic origin, have shown promise in achieving these aims; these techniques are now being explored clinically in patients as an alternative for nerve graft repair, though their clinical use has been limited mainly to the repair of relatively small defects (<3 cm) and in small-caliber digital nerves.45 These techniques are also supplemented with neurolysis, in which release of perifascicular and perineural adhesions is performed. A collagen-based sheath referred to as a “nerve wrap” may also be used to prevent recurrent adhesions around the neurolyzed nerve segment.46
Specific cases are highlighted below to demonstrate how the surgical correlation of these small peripheral nerve lesions may be surmised from the imaging appearance on high-resolution MRN.
Neurapraxia
Neurapraxia represents the mildest injury and may result from direct trauma, traction, friction, compressive lesion, or iatrogenic causes. While rare, lower extremity nerve injury due to patient positioning and compression during surgery has been reported. Sciatic nerve deficits and piriformis syndrome have been described after surgery in lithotomy or sitting positions.47–49 CPN is another peripheral nerve in the lower limb that is vulnerable to injury, especially at the neck of the fibula, due to operative positioning, particularly for hip surgery.50,51 Regardless of the cause, nerve injuries at the level of neurapraxia may give rise to abnormal findings of nerve pathology and regional muscle denervation on MRN as discussed above. Most cases resolve with conservative treatment.
Axonotmesis and Neuroma Formation
Nerve injury involving fascicular disruption may be seen with axonotmesis (Fig 3) and sometimes, there may be formation of a neuroma, which has the characteristic MRN findings of discrete nodular or fusiform nerve enlargement. While injury-related neuromas may exhibit variable-to-no enhancement, more avid enhancement may help to distinguish nerve sheath tumors from posttraumatic neuromas.13 Perineural fibrosis of the interdigital nerves of the foot (ie, so-called Morton neuroma) may also show variable enhancement.52 MR imaging of the foot has a well-established role in the diagnosis of the Morton neuroma.53 Following resection, high-quality MRN may depict residual neuroma as persistent nodular enhancement as well as additional unsuspected neuromas (Fig 4).
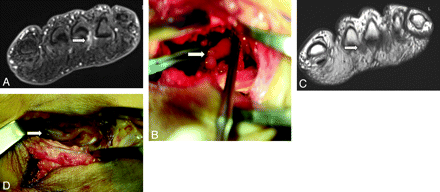
MRN appearance of a Morton neuroma (interdigital neuroma or perineural fibrosis). A 42-year-old man presented with persistent neuropathic pain after resection of the common digital nerve in the third web space of the foot. A, Short-axis T1WI after IV injection of gadolinium shows focal enhancement in the third webspace, consistent with postoperative changes and a small residual neuroma (arrow). B, Intraoperative photograph confirms the residual neuroma in the third webspace (arrow). C, Short-axis T1WI of the same patient demonstrates an additional 6-mm lesion in the second webspace, representing another clinically unsuspected interdigital neuroma (arrow). D, Intraoperative photograph confirms the additional interdigital neuroma in the third webspace (arrow).
Neurotmesis
Neurotmesis may result from penetrating trauma (Fig 5), crush injury, or iatrogenic causes such as injury to the sciatic or femoral nerve during hip replacement.54–56 As with axonotmesis, any injury at the level of neurotmesis may lead to neuroma formation. MR imaging in cases of intraoperative nerve injury can be challenging if there is metallic hardware in the area of injury. Multiple techniques may be used to increase the SNR, such as decreased echo-train length, decreased TE, acquisition of multiple averages, wider receiver bandwidth, and swapped frequency and phase-encoding directions.57,58 Metal-related image distortion may be minimized with fast spin-echo sequences.59 With the use of these strategies, high-resolution images may be obtained even in an area close to the metallic hardware (Fig 6).
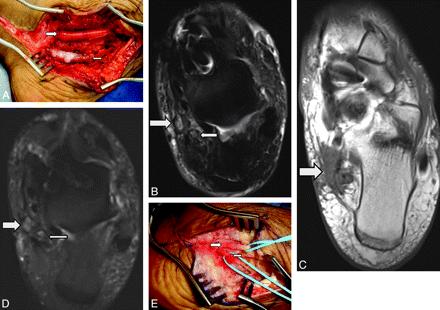
MRN appearance of an abnormal neurotomized CPN. A 42-year-old woman presented with progressively worsening foot drop following penetrating trauma, which occurred about 2 months earlier. A, Axial T2 SPAIR image at the level of the popliteal fossa shows a thickened perineurium and abnormal T2 hyperintensity of the peroneal nerve (arrow). Note the disrupted fascicular appearance of the nerve (arrow). Regional denervation muscle edema and atrophy in extensor compartment muscles were also seen (not shown). B, Oblique reconstruction from a 3D T2 SPACE sequence along the axis of the injured CPN demonstrates an enlarged hyperintense CPN with neurotmesis and fibrosis distally.
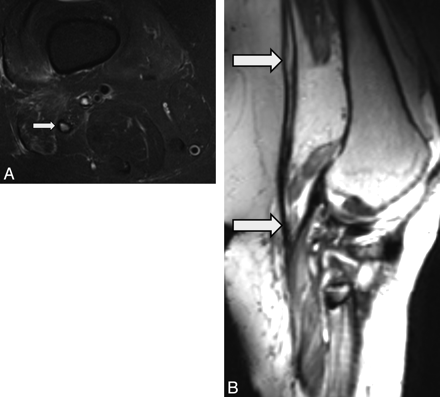
MRN appearance of an abnormal sciatic nerve with neurotmesis. A 50-year-old woman presented with foot drop following left hip-replacement surgery (metallic hardware) performed 6 months earlier. A, Coronal 3D T2 SPACE image shows focal discontinuity of the sciatic nerve (large arrow) with proximal atrophy (small arrow). Neurotmesis of the sciatic nerve was confirmed during surgery, and the nerve was repaired. B, Intraoperative photograph shows the sciatic nerve after repair with a cable sural nerve autograft (arrows), which bridges the torn ends of the sciatic nerve.
Failed Peripheral Nerve Surgery
Failed peripheral nerve surgery (eg, persistent or recurrent symptoms following carpal, tarsal, or cubital tunnel release) is another indication for MRN because clinical evaluation and electrodiagnosis may be challenging in these cases.60,61 MRN plays a vital role in the preoperative evaluation of “redo nerve release” because it may demonstrate residual or recurrent pathology, complications of previous surgery (such as hematoma, abscess, focal perineural encasing fibrosis), or additional unexpected findings, such as tenosynovitis or plantar fasciitis, which could be responsible for persistent symptoms. MRN may thus help to create a roadmap for the surgeon before a redo surgery (Fig 7 ). On MRN, collagen-based nerve conduits, such as nerve tubes and wraps, are seen as curvilinear hypointensities on both T1WI and T2WI (Fig 7D, -E).5 Sequential MRN imaging may show resorption of collagen-based nerve conduits. In cases with good treatment response, normalization of nerve and muscle SI may be seen with time. MRN may also be used as a noninvasive tool to follow these cases after redo surgery, especially if the initial outcome is not satisfactory (Fig 8 ). Nonresponding cases may show further worsening of T2 SI within the nerves and innervated muscles, along with fatty replacement and atrophy of the muscles on sequential MR imaging.62 These MRN findings should be interpreted in an appropriate clinical context, while one takes into account all available information from clinical history, focused neurologic examination, and electrodiagnostic tests.
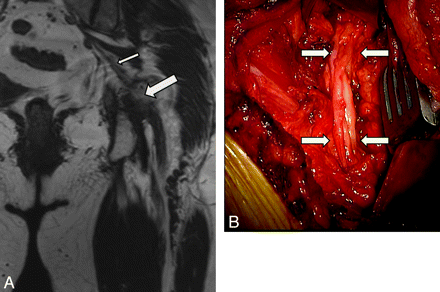
MRN appearance of abnormal MPN and LPN in a 32-year-old woman with persisting and worsening heel pain and toe weakness after a tarsal tunnel release surgery. A, Coronal oblique T2 SPAIR image obtained before redo surgery shows abnormally enlarged and T2 hyperintense MPN (larger arrow) and LPN (smaller arrow) in the tarsal tunnel. Note the mild denervation edema of the abductor hallucis (curved arrow). B, Intraoperative photograph during neurolysis confirms the MRN findings of abnormal MPN (larger arrow) and LPN (smaller arrow) in the distal tarsal tunnel. C, Another intraoperative photograph shows both the MPN (larger arrow) and LPN (smaller arrow) as well as the tibial nerve (curved arrow). All 3 nerves were placed in collagen-based nerve wraps. The patient, however, continued to have pain and was referred for MRN about 6 weeks following the redo tarsal release. D, Coronal oblique T1WI demonstrates markedly enlarged MPN (larger arrow) and LPN (smaller arrow). Both nerves are encased in exuberant fibrosis and show loss of the fascicular pattern as well. E, Coronal oblique T2 SPAIR image shows worsening neuropathy of MPN (larger arrow) and LPN (smaller arrow), indicated by further enlargement and increased T2 hyperintensity of both nerves. Worsening muscle denervation edema is also nicely depicted (curved arrows in E). In D and E, previously placed nerve wraps are seen as hypointense structures encircling both the medial and plantar nerves.
MRN appearance of an abnormal MPN and LPN in a 48-year-old woman who was referred for MRN due to persistent pain in the foot. She had undergone tarsal tunnel release, neurolysis, nerve tube, and wrap placement. A, Intraoperative photograph shows the MPN in a nerve wrap (larger arrow) and the LPN in a nerve tube (smaller arrow). B, Axial T2 SPAIR image from the MRN obtained 6 months after redo surgery shows abnormal T2 hyperintensity and enlargement of both the MPN (larger arrow) and LPN (smaller arrow). Note the circumscribed rim of T2 hypointensity around the MPN and LPN related to nerve wrap and nerve tube materials. C, Axial T1WI distally shows extensive fibrosis (arrow) below the level of abductor hallucis, not addressed during initial neurolysis, causing persistent distal entrapment of the MPN and LPN. The patient continued to have symptoms and another MRN was performed 11 months after redo surgery. D, Oblique coronal STIR image more distally shows worsening increasing size and hyperintensity of the MPN (larger arrow) and LPN (smaller arrow), compared with prior MRN. Persistent muscle denervation changes were again noted (not shown). E, Intraoperative photograph, about a year from the first redo tarsal tunnel surgery. The patient underwent another tarsal tunnel release surgery, which confirmed MRN findings of encasing perineural fibrosis. Notice the inflamed and hyperemic MPN (larger arrow) and LPN (smaller arrow) following neurolysis. The previously placed nerve wrap and nerve tube are resorbed.
Nerve Regeneration
An additional application of MRN, currently in the early stages of evaluation, is the imaging of nerves undergoing regeneration. Nerve conduits (nerve tubes) are increasingly being used for the repair of relatively small defects (<3 cm) in small-caliber digital nerves. The nerve tube is sutured to freshened edges of the nerve in the hope of promoting nerve regeneration. Reported rates of nerve regeneration vary from 0.5 to 1 mm per day.63 During the immediate postoperative period, an empty nerve tube is filled with fluid and accordingly demonstrates marked T1 and T2 SI prolongation (Fig 9 A). Early nerve regeneration and nerve sprout formation within nerve tubes may manifest on MRN as tiny filling defects within the conduit (Fig 9B). In our limited anecdotal experience, these filling defects develop a fascicular appearance with increasing T2 SI during 2–4 months, best seen on T2WI. The eventual fate of these multiple sprouts is not always clear, even in experimental paradigms, and distal unification may not occur. The regeneration and repair phase following nerve injury may last for many months up to a year; moreover, axonal regeneration does not always correlate with return of function. Thus, further randomized controlled and larger studies are needed to evaluate the potential of high-resolution T2- and diffusion-based MRN examinations, to optimally evaluate the nerve regeneration response, as correlated with clinical and electrophysiologic tests. This may lead to better understanding, diagnosis, and treatment of peripheral nerve disease.
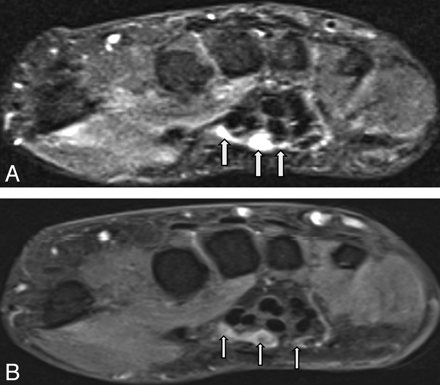
MRN appearance of regeneration of a peripheral nerve. A 46-year-old woman with a prior median nerve injury underwent repair and nerve tube placement across median nerve branches. A, Axial STIR image shows fluid-filled nerve tubes (small arrows). B, Axial T1WI obtained after IV administration of gadolinium shows filling defects within the fluid-filled nerve tubes (small arrows). These appear as nonenhancing hypointense structures or filling defects within the tubes, which were confirmed as hypertrophied nerve sprouts that failed to unite at surgery.
Summary
High-resolution MRN techniques improve the visualization of normal and pathologic peripheral nerves and supplement information gained from clinical findings and electrophysiologic tests. MRN assists in the diagnosis of peripheral nerve pathology, creates a preoperative roadmap for the peripheral nerve surgeon, and serves as a useful technique for postoperative follow-up.
Footnotes
-
T.K. Subhawong was supported by grant 1T32EB006351 from the National Institutes of Health. Kenneth Wang acknowledges the support of Research and Education Foundation Fellowship Training Grant FT0904 of the Radiological Society of North America, as well as that of the Walter and Mary Ciceric Research Award. Avneesh Chhabra acknowledges the support of research grants from Siemens AG ($50,000 grant to study MR neurography) and Integra Life Sciences as well as the support of a GE-AUR Fellowship. John A. Carrino acknowledges a $50,000 grant from Siemens Medical Systems to study MR neurography.
-
Paper previously presented at: Annual Meeting of the American Roentgen Ray Society, May 2–7, 2010; San Diego, California.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.↵
- © 2012 by American Journal of Neuroradiology