SUMMARY:
CNS lymphoma consists of 2 major subtypes: secondary CNS involvement by systemic lymphoma and PCNSL. Contrast-enhanced MR imaging is the method of choice for detecting CNS lymphoma. In leptomeningeal CNS lymphoma, representing two-thirds of secondary CNS lymphomas, imaging typically shows leptomeningeal, subependymal, dural, or cranial nerve enhancement. Single or multiple periventricular and/or superficial contrast-enhancing lesions are characteristic of parenchymal CNS lymphoma, representing one-third of secondary CNS lymphomas and almost 100% of PCNSLs. New CT and MR imaging techniques and metabolic imaging have demonstrated characteristic findings in CNS lymphoma, aiding in its differentiation from other CNS lesions. Advanced imaging techniques may, in the future, substantially improve the diagnostic accuracy of imaging, ultimately facilitating a noninvasive method of diagnosis. Furthermore, these imaging techniques may play a pivotal role in planning targeted therapies, prognostication, and monitoring treatment response.
Abbreviations
- ADC
- apparent diffusion coefficient
- CBV
- cerebral blood volume
- CE
- contrast enhancement
- Cho
- choline
- CNS
- central nervous system
- Cr
- creatine
- DWI
- diffusion-weighted imaging
- FA
- fractional anisotropy
- FDG
- fluorodeoxyglucose
- HAART
- highly active antiretroviral therapy
- HIV
- human immunodeficiency virus
- MRI
- MR imaging
- MS
- multiple sclerosis
- NHL
- non-Hodgkin lymphoma
- PCNSL
- primary CNS lymphoma
- PET
- positron-emission tomography
- PML
- progressive multifocal leukoencephalopathy
- rCBV
- relative cerebral blood volume
- SPECT
- single-photon emission CT
- SPET
- single photon-emission tomography
- SWI
- susceptibility-weighted imaging
Lymphoma of the CNS consists of 2 major subtypes: secondary CNS involvement by systemic lymphoma (the most common) and PCNSL, in which the lymphoma is restricted to the brain, leptomeninges, spinal cord, or eyes, without evidence of it outside the CNS at primary diagnosis.1,2
The frequency of secondary CNS lymphoma in patients with systemic lymphoma varies and is highly dependent on histologic subtype. The overall risk of CNS relapse in aggressive NHL is on the order of 2%–27%,3,4 whereas patients with Hodgkin lymphoma are at very low risk (≤0.5%).3 Patients with extranodal involvement and those with primary or acquired immunodeficiency disorders carry an increased risk of CNS relapse.3
PCNSL accounts for 1%–5% of all brain tumors5,6 and approximately 1% of all NHLs.5 The incidence rates of PCNSL are increasing among immunocompetent patients.5,7,8 Immunocompromised patients (eg, individuals infected with HIV) have an increased risk of developing PCNSL.9 However, with the introduction of HAART during the past decade, the incidence of PCNSL in the HIV population has declined.10,11
Early diagnosis of CNS lymphoma is crucial for proper management in both immunocompetent and immunocompromised individuals and is more likely if a tumor is observed on imaging.12,13 Although CNS lymphomas may have characteristic imaging findings on traditional CT and MR imaging, none of these will unequivocally differentiate CNS lymphoma from other brain lesions.9,14–16 A visible tumor on imaging is essential to raise the suspicion of CNS lymphoma, which then can lead to an early histologic diagnosis based on cytology of the CSF or brain biopsy.
This pictorial essay reviews some typical imaging features at the presentation of CNS lymphoma on traditional imaging. Characteristic imaging findings with newer advanced imaging techniques that may potentially aid in the differentiation of CNS lymphoma from other brain lesions are also discussed.
Secondary CNS Lymphoma: Traditional Imaging
CNS involvement in aggressive NHL tends to occur early at a median of 5–12 months after the primary diagnosis of NHL.3 Approximately two-thirds of the patients present with leptomeningeal spread and one-third, with parenchymal disease.3 Leptomeningeal spread, similar to leptomeningeal metastases from any cause, often involves the cranial nerves, spinal cord, or spinal roots and may present as cranial or spinal neuropathies.17 Headache is present in 30%–40% of patients with leptomeningeal metastases,18 which may be due to increased intracranial pressure from metastatic obstruction of CSF flow/absorption.17
The imaging technique of choice to detect leptomeningeal metastasis is contrast-enhanced MR imaging.17,18 CT is less sensitive,18 especially in patients with hematologic malignancies.19 Neuroimaging findings suggestive of leptomeningeal metastases include leptomeningeal, subependymal, dural, or cranial nerve enhancement; superficial cerebral lesions; and communicating hydrocephalus (Table 1).17,18,20,21 Lumbar puncture may induce dural enhancement in the cranial or spinal space, which may be falsely interpreted as leptomeningeal enhancement. Lumbar puncture should, therefore, be avoided before neuroimaging.18
Typical imaging features of primary and secondary CNS lymphoma
Parenchymal metastases from NHL often appear as single or multiple enhancing lesions and can be accompanied by leptomeningeal metastases.17,21 The parenchymal lesions may have a periventricular and/or superficial location (Fig 1). The typical MR imaging findings are quite similar to the findings in PCNSL; this similarity makes it impossible to discriminate these 2 entities on the basis of neuroimaging.16 As for all primary brain tumors and brain metastases, MR imaging is the imaging technique of choice for the detection of lesions and preoperative planning of surgical diagnostic procedures.
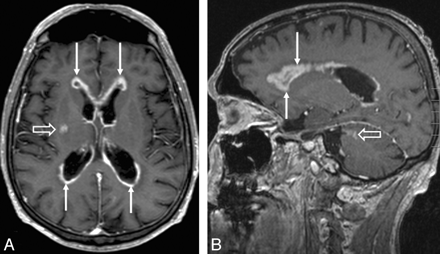
Axial (A) and sagittal (B) T1-weighed contrast-enhanced MR images in a patient with CNS metastases from NHL show diffuse subependymal contrast enhancement (arrows) and 2 parenchymal lesions (open arrows) in the right basal ganglia (A) and left cerebellum (B).
Approximately 50% of the patients with CNS metastases from NHL have progressive systemic lymphoma at the time of diagnosis of their CNS manifestation.3 Furthermore, most of the remaining 50% of patients with apparently isolated CNS metastases develop systemic disease within months.3 Systemic manifestations of NHL thus commonly accompany the findings of parenchymal or leptomeningeal disease; these manifestations normally raise clinical suspicion of CNS metastases and lead to early cytology or brain biopsy to confirm the diagnosis.
PCNSL: Conventional CT and MR Imaging
PCNSL often has a characteristic appearance on both CT and MR imaging (Table 1). This is due to its hypercellularity, high nuclear/cytoplasmic ratio, disruption of the blood-brain barrier, and its predilection for the periventricular and superficial regions, often in contact with ventricular or meningeal surfaces.22–25
In both immunocompetent and immunodeficient patients with PCNSL, unenhanced CT typically reveals hyper- or isoattenuated lesions, and virtually all lesions show contrast enhancement.24–27 Negative CT findings, however, do not exclude PCNSL because false-negative initial CT examinations are also reported.14,25 On unenhanced T1-weighted MR imaging, lesions are typically hypo- or isointense,14,24–27 and on T2-weighted MR imaging, iso- to hyperintense but often hypointense to gray matter.14,25,26,28 Most lesions show moderate-to-marked contrast enhancement on CT and MR imaging.14,15,24–27,29 Isolated white matter hyperintensity on T2-weighted MR imaging or no contrast enhancement on T1-weighted MR imaging has also been described in some rare cases of PCNSL.14,30
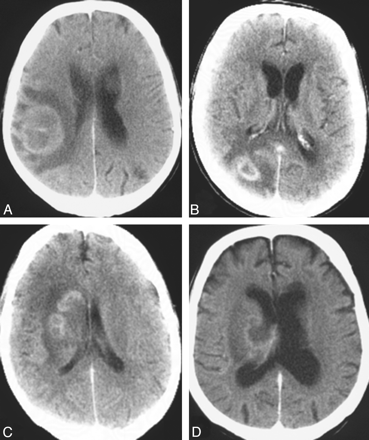
Non-AIDS PCNSL typically presents as a solitary homogeneously enhancing parenchymal mass (Fig 2).14 Multiple lesions are reported in 20%–40% of non-AIDS PCNSLs,14,15,27,29,31 and ringlike enhancement, in 0%–13% (Figs 2 and 3).14,29,31,32 Linear enhancement along perivascular spaces is highly suggestive of PCNSL.23,25 Perifocal edema is usually present but less prominent than that in malignant gliomas or metastases.14,33 Most lesions are located in central hemispheric or periventricular cerebral white matter.14,25,34 A superficial location adjacent to the meninges is also common. Frontal lobe location is reported in 20%–43% of PCNSLs, whereas the basal ganglia are affected in 13%–20%.14,26,32,35 The brain stem or cerebellum or both are affected in 9%–13%,15,32,35 and the spinal cord, in only 1%–2% of patients.15,29 Hemorrhage or internal calcification within the tumor is quite a rare finding.14,27
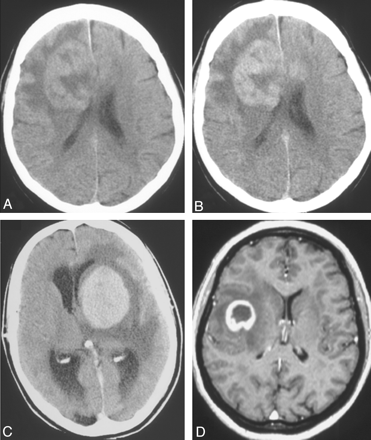
Solitary lesions on a noncontrast CT scan (A), contrast-enhanced CT scans (B and C), and an MR image (D) in 3 patients with non-AIDS PCNSL. Note a hyperattenuated lesion in the frontal lobe on the noncontrast CT scan (A) with marked enhancement on the contrast series (B) and focal contrast-enhancing lesions in the left basal ganglia (C) and temporal lobe (D). One lesion has ring enhancement (D).
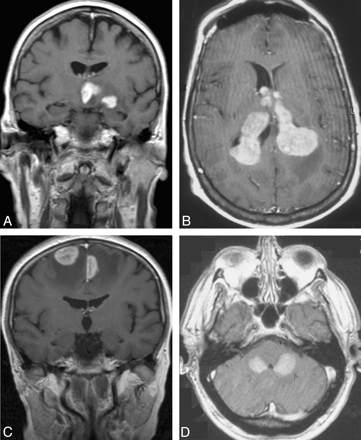
Multiple lesions on contrast-enhanced axial (B and D) and coronal (A and C) MR images in 4 patients with non-AIDS PCNSL. Note lesions in the basal ganglia (A), ventricles (B), frontal lobes (C), and cerebellar lobes (D).
An extension of parenchymal PCNSL to the eye, denoting secondary intraocular lymphoma, may be asymptomatic and has been reported in 25% of the PCNSLs.36 It may be diagnosed by cytologic examination of vitreal aspirate or by slit-lamp examination.13,23 Primary intraocular lymphoma, the lymphoma being solely restricted to the eye, is a very rare subset of PCNSL.13 Using a dedicated thinsection MR imaging protocol, intraocular lymphoma may be detected as nodular contrast-enhancing lesions at the macula or thickening of the uvea.37 However, MR imaging has a low sensitivity for intraocular lymphoma, and negative findings do not preclude the intraocular involvement of PCNSL.37
Primary dural lymphoma is a rare subtype of PCNSL that differs biologically from other PCNSLs because it arises from the dura mater.38 CT or MR imaging typically reveals diffusely enhancing single or multiple extra-axial masses, which often mimic meningiomas (Fig 4).38 The cerebral convexities are most commonly involved; however, dural lymphoma may also be found in the falx, tentorium, sellar/parasellar regions, or spine.38
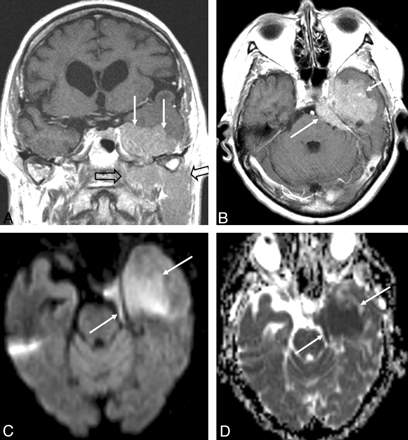
Coronal (A) and axial (B) contrast-enhanced T1-weighted MR images and an axial DWI (C) and ADC map (D) in a patient with primary dural B-cell lymphoma (arrows) with tumor spread below the skull base (open arrow). The contrast-enhancing tumor at the caudal surface of the left temporal lobe (A and B) displaces the temporal lobe cranially and resembles a meningioma. DWI reveals restricted diffusion within the tumor with high signal intensity on DWI (C) and corresponding low signal intensity on the ADC map (D).
Immunodeficient patients with PCNSL are often diagnosed with multifocal lesions, which are reported in 30%–80% of patients with AIDS-related PCNSL (Fig 5).28,31,39 Because many lesions exhibit necrotic regions, contrast enhancement is commonly irregular or peripheral, and ringlike enhancement is reported in up to 75% of cases.28,31,39 The basal ganglia and corpus callosum are frequently involved.28,34,39 Spontaneous hemorrhage in PCNSL lesions may be more frequent in AIDS patients than in non-AIDS patients.28
Contrast-enhancing lesions on CT scans (A–D) in 4 patients with AIDS-related PCNSL. Note irregularly enhancing lesions in the right parietal lobe (A), right occipital lobe (B), and right periventricular white matter (C and D); most of the lesions show ring enhancement (A, B, and C).
CNS Lymphoma: Advanced CT and MR Imaging
CNS lymphomas may have a characteristic appearance on traditional CT and MR imaging; however, none of these imaging characteristics will unequivocally differentiate CNS lymphomas from other neoplasms (eg, metastases from other malignancies, malignant gliomas, meningiomas) or non-neoplastic diseases (eg, multiple sclerosis, stroke, cerebral toxoplasmosis, pyogenic abscess).9,14–16 Furthermore, the typical imaging characteristics may not be present.14 DWI,25,40 perfusion MR imaging,40,41 and MR spectroscopy42,43 are increasingly used in clinical radiologic practice and may help to differentiate CNS lymphomas from other lesions of the brain (Table 2).
Advanced imaging techniques in CNS lymphoma
DWI measures the diffusion of water molecules in biologic tissues; diffusion within the tumor is considered a surrogate marker of tumor cellularity because intact cells constitute a barrier to water diffusion.44 Because CNS lymphomas are highly cellular tumors, water diffusion is often restricted, making them appear hyperintense on DWI and hypointense on ADC maps (Figs 4 and 6).16,40,44,45 This characteristic is shared by acute ischemic stroke, the central necrosis of brain abscesses, the solid portion of high-grade gliomas, and some metastases.45 However, PCNSL lesions often have more restricted diffusion and lower ADC values than high-grade gliomas and metastases.40,46,47
A recent study showed that pretherapeutic ADC tumor measurements within contrast-enhancing regions were predictive of clinical outcome in patients with PCNSL.48 Low ADC values were predictive of shorter progression-free survival and overall survival. In addition, an inverse correlation was found between ADC values and the cellular density of the tumors. Patients with prolonged progression-free survival and overall survival also had a significant reduction in post-therapeutic ADC values.48 Thus, repeated ADC measurements may be used as biomarkers in the surveillance of therapeutic response.
Diffusion tensor imaging requires diffusion measurements in at least 6 directions and is a sensitive tool for the detection of alterations in white matter structure.47 A quantitative FA map shows hypointensity corresponding to decreased FA values in most brain tumors.47 Different degrees of cellularity and cellular organization may also affect the FA value, and FA values of PCNSL are significantly lower than those of glioblastoma multiforme, aiding in the differentiation of these tumors.47
The documented importance of revascularization through angiogenesis for tumor growth has led to a growing interest in novel imaging techniques to assess tumor vascularity. Perfusion MR imaging and perfusion CT visualize nutritive delivery of arterial blood to the capillary bed in the biologic tissue (eg, tumors); postprocessing of the acquired data enables calculation of physiologic parameters, such as CBV, cerebral blood flow, mean transit time, and time to peak.49–51 PCNSLs demonstrate low CBV (Fig 6) and a characteristic intensity time curve, which is related to a massive leakage of contrast media into the interstitial space.41 Furthermore, maximum relative CBV measured in tumor tissue, calculated as a ratio to contralateral normal-appearing white matter, is typically lower in lymphomas than in other brain tumors. This characteristic finding can help to differentiate glioblastomas and metastases from lymphomas.40,52
Axial contrast-enhanced T1-weighted MR image (A), sagittal T2-weighted MR image (B), axial DWI (C), an ADC map (D), and an rCBV map (E) in a patient with PCNSL. The periventricular contrast-enhancing tumor (A) in the left parietal lobe has restricted diffusion with high signal intensity on DWI (C) with corresponding low signal intensity on the ADC map (arrows, D). Perfusion MR imaging shows low perfusion within the contrast-enhancing tumor on the rCBV map (arrows, E).
Both perfusion MR imaging and perfusion CT may demonstrate increased microvascular permeability in tumor tissue based on quantification of the permeability surface area product and the contrast transfer coefficient.50,53 Several studies have reported that these parameters measured on MR imaging correlate with the mitotic index, histologic grading, and biologic aggressiveness of gliomas, but this correlation has not yet been investigated with regard to CNS lymphomas.50 In contrast to MR imaging, only a few studies on perfusion CT in brain tumors50,51 have been published, possibly due to concern about the radiation dose with CT and potential nephrotoxicity of iodinated contrast agents.50 However, the diagnostic value of perfusion CT in brain tumors may become more important in the future because it offers advantages over perfusion MR imaging in terms of spatial resolution, insensitivity to paramagnetic susceptibility artifacts, and linear correlation between contrast concentration and attenuation.50
MR spectroscopy obtains biochemical information noninvasively from biologic tissue. Within a defined volume of interest, signals may be registered from chemical nuclei within the body; the most commonly used nuclei are protons (hydrogen). In PCNSL, proton MR spectroscopy has demonstrated elevated lipid peaks combined with high Cho/Cr ratios.29,42–44 These can, however, also be seen in glioblastoma multiforme42 and metastases but may help in differentiating PCNSL from other lesions.
High-resolution SWI is much more sensitive than conventional MR imaging for the visualization of small veins, blood products, and calcifications, which appear as low-signal intensity structures.54 This is helpful in differentiating PCNSL from high-grade gliomas, because microhemorrhages and calcifications are rare in PCNSL, whereas small hemorrhages are frequently seen in high-grade gliomas.54
More recently, superparamagnetic contrast agents, (eg, iron oxide nanoparticles) have become available.55 MR imaging with iron oxide nanoparticles may help to distinguish malignant neoplasms from multiple sclerosis56; however, to our knowledge, the potential role of this technique in the diagnosis of CNS lymphoma has not yet been defined.
In AIDS patients, DWI cannot reliably differentiate CNS lymphomas from cerebral toxoplasmosis because they show overlapping ADC ratios.45 Perfusion MR imaging has also been disappointing in this respect.57 However, on MR spectroscopy, toxoplasmosis, lymphoma, and PML brain lesions display distinctly different biochemical profiles,58,59 aiding in correctly diagnosing 94% of the lesions in 1 study.59
CNS Lymphoma: Metabolic Imaging
Medical nuclear imaging techniques by using radioisotopes to produce images that reflect biologic processes are examples of metabolic imaging. PET with FDG typically reveals hypermetabolic lesions with an increased uptake of FDG in CNS lymphomas25 and may help to identify and differentiate lymphomas from malignant gliomas and meningiomas (Table 2).60 PCNSLs show more pronounced metabolic activity than do metastases and high-grade gliomas.25 Furthermore, FDG-PET may be suitable for early evaluation of a therapeutic response.60 After steroid treatment, the degree of hypermetabolic activity in PCNSL may decrease.61
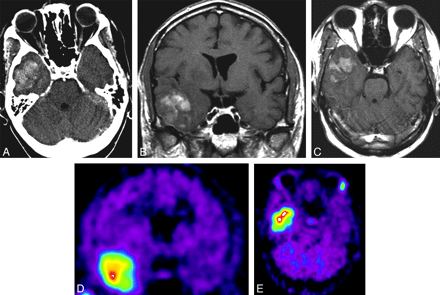
PET performed with 11C-methionine (methionine PET) shows very high uptakes in CNS lymphomas, which corresponds to the enhancing portion on CT/MR imaging, and the area of increased uptake is often larger than the enhancing lesions (Fig 7).62 This larger area of methionine uptake reflects tumor infiltration beyond the enhancing portion seen on MR imaging and CT.62 The size and degree of methionine accumulation in the tumor tissue decrease following radiation therapy.62 Thus, methionine PET may provide a more accurate delineation of tumor volume for the evaluation of the therapeutic effect of radiation therapy as well as for the detection of residual or recurrent tumor after treatment.62 SPECT in non-AIDS PCNSL has shown high iodine 123 N-isopropyl-p-iodoamphetamine retention in CNS lesions, aiding in the diagnosis of PCNSL.63
Axial contrast-enhanced CT scan (A), coronal (B) and axial (C) contrast-enhanced T1-weighted MR images, and methionine PET (coronal, D, and axial, E) images in a patient with PCNSL. The contrast-enhancing tumor in the right temporal lobe (A–C) shows high uptake of methionine on PET (D and E).
Among immunocompromised individuals, infectious lesions in the CNS are usually hypometabolic, whereas lesions caused by CNS lymphomas are hypermetabolic, with a high thallium-201 uptake ratio on SPECT and SPET9,64 and high FDG uptake on PET.9 These characteristics may aid in the differentiation of infectious intracranial lesions and CNS lymphomas in AIDS patients.9,57,64
Future Perspectives
New PET, SPECT, and SPET tracers as well as new MR imaging contrast agents that may potentially reveal important aspects of tumor biology are currently being intensively investigated by researchers all over the world.55,65 This research may provide new insights that will substantially improve the preoperative diagnostic accuracy of imaging, enabling an appropriate diagnostic procedure as well as early active treatment in this patient group. In the future, improved advanced imaging techniques may noninvasively provide an accurate diagnosis, obviating surgical biopsy before the initiation of chemotherapy, radiation therapy, and new nonsurgical therapeutic regimens. An integrated PET/MR imaging system has recently been developed that gives simultaneous morphologic and biologic information, creating new possibilities for complementary information.66 Some of these newer imaging techniques will presumably play a pivotal role in the planning of new targeted therapies, in monitoring treatment response, and in the prediction of treatment outcomes.
Conclusions
When CNS lymphoma is suspected, contrast-enhanced MR is the imaging technique of choice. Secondary CNS lymphomas present as leptomeningeal metastases in two-thirds of the patients and as parenchymal metastases in one-third. In PCNSL, almost all patients have parenchymal lesions. Parenchymal lymphomas have a predilection for the periventricular and superficial regions, often abutting the ventricular or meningeal surfaces. Although CNS lymphomas may have characteristic imaging findings on traditional MR imaging, none of these will unequivocally differentiate CNS lymphoma from other brain lesions.
New advanced MR imaging techniques and PET and SPECT metabolic imaging have identified characteristic findings in CNS lymphoma that may aid in the differentiation of CNS lymphomas and other CNS lesions. In the future, improved advanced imaging techniques may provide morphologic and biologic information noninvasively and, thus, an accurate diagnosis. Furthermore, these imaging techniques will presumably play an important role in the planning of new targeted therapies, for prognostication, and for the monitoring of treatment response.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.↵
- 26.↵
- 27.↵
- 28.↵
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.
- 45.
- 46.
- 47.
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.
- 57.↵
- 58.↵
- 59.
- 60.
- 61.↵
- 62.
- 63.
- 64.
- 65.↵
- 66.↵
- Copyright © American Society of Neuroradiology