Abstract
SUMMARY: The relevant aspects of cholesteatomas are reviewed with the emphasis on their diagnosis by using cross-sectional imaging. The indications and limitations of CT and MR imaging and the use of novel MR imaging techniques in the diagnosis of cholesteatomas are described. HRCT of the temporal bone has an excellent spatial resolution, thus even small soft-tissue lesions can be accurately delineated (high sensitivity). However, CT has poor specificity (ie, soft-tissue structures cannot be differentiated). MR imaging with the conventional sequences (T1WI, T2WI, postcontrast T1WI) provides additional information for distinguishing different pathologic entities and for accurately diagnosing primary (nonsurgical) and residual/recurrent (surgical) cholesteatomas. Higher diagnostic specificity is achieved by introducing DW-EPI, delayed postcontrast imaging, DW-non-EPI, and DWI-PROPELLER techniques. Studies using DW-non-EPI and DWI-PROPELLER sequences show promising results related to improved diagnostic sensitivity and specificity for even small (<5 mm) cholesteatomas, thus allowing avoidance of second-look surgery in the future.
Abbreviations
- DWI
- diffusion-weighted imaging
- DW-EPI
- diffusion-weighted echo-planar imaging
- DWI-PROPELLER
- diffusion-weighted imaging with periodically rotated overlapping parallel lines with enhanced reconstruction
- DW-non-EPI
- diffusion-weighted non-echo-planar imaging
- EAC
- external auditory canal
- EACC
- external auditory canal cholesteatoma
- EPI
- echo-planar imaging
- FIESTA
- fast imaging employing steady-state acquisition
- FLASH
- fast low-angle shot
- HRCT
- high-resolution CT
- NPV
- negative predictive value
- PPV
- positive predictive value
- T1WI
- T1-weighted imaging
- T2WI
- T2- weighted imaging
- TM
- tympanic membrane
Cholesteatoma has been known for more than 300 years in the medical literature; still its precise detection with the use of cross-sectional imaging techniques remains challenging. As before, the diagnosis of a cholesteatoma at first presentation is mainly based on clinical suspicion. HRCT provides information about bony changes and intracranial complications; however, it is inaccurate for characterizing the soft-tissue mass in the temporal bone. In the past 7 years, improvements in MR imaging techniques have enhanced the sensitivity and specificity of radiologic diagnosis, which may lead to future avoidance of second-look surgeries in cases of residual/recurrent cholesteatomas.
The purpose of this review article is to summarize all aspects of cholesteatomas, including their definition, history, etymology, epidemiology, classification, histology, pathophysiology, clinical signs, and neuroradiologic diagnosis. We review the latest studies on the application of new MR imaging techniques for the accurate diagnosis of cholesteatomas.
Definition
“Cholesteatoma” is a well-demarcated non-neoplastic lesion in the temporal bone, which is commonly described as “skin in the wrong place.”1
History and Etymology
Joseph-Guichard Duverney, a French anatomist, was the first to describe a temporal bone lesion in 1683, probably representing a cholesteatoma.2 In 1838, this pathology was named “cholesteatoma” (Greek: chole + stear = fat, oma = tumor) by the German anatomist/pathologist Johannes Müller.3 However, this term is incorrect because the lesion does not contain fat and is not of a neoplastic nature.
Although other more descriptive denominations were suggested, such as “pearl tumor,” “margaritoma,” or “keratoma,” the most commonly used expression is the misnomer “cholesteatoma.”
Epidemiology
The annual incidence of cholesteatoma is reported as 3 per 100 000 in children and 9.2 per 100 000 in adults with a male predominance of 1.4:1. Middle ear cholesteatomas have a higher incidence in individuals younger than 50 years of age, whereas EAC cholesteatomas present predominantly at 40–70 years of age. Hereditary predisposition is probable. There is a high prevalence among white individuals, and cholesteatoma is rarely detected in the Asian, American Indian, and Alaskan Eskimo populations.
Classification
The most widely used classifications for cholesteatomas are based on either their pathogenesis or on their location in the middle ear cavity in relation to the TM. The different taxonomies reveal some overlapping, which—by reviewing the literature—makes the comprehensibility difficult. Additionally, we refer to 2 special groups of cholesteatomas as well: “mural” cholesteatomas and EACCs.
Classification Based on Pathogenesis
Cholesteatomas can be classified as either congenital or acquired,4 though the origins are indistinguishable with histology and imaging. Only the location of the lesion, the clinical history of the patient, and the otologic status of the TM give some hints for differentiating these 2 types of cholesteatomas (Table 1).
Classification of all cholesteatomas based on pathogenesis
“Congenital cholesteatomas” develop from embryonic epithelial rests and can be located everywhere in the temporal bone: in the middle ear (Fig 1), in the mastoid, in the petrous apex (Fig 2), in the squama of the temporal bone, within the TM, or in the EAC (Fig 3). Furthermore, the same histologic entity can arise in other areas of the skull, in the extracranial soft tissues, or in an intracranial extra-axial location, where it is referred to as “epidermoid cyst.” Middle ear congenital cholesteatomas represent approximately 2% of all middle ear cholesteatomas.
Acquired cholesteatomas are further subdivided and are uniquely localized in the middle ear.
Congenital cholesteatoma. Coronal (A) and axial HRCT (B) scans demonstrate a round well-defined lesion (arrow) anterosuperior in the tympanic cavity, medial to the ossicular chain. Note the missing ossicular erosion. Based on the position of the lesion and the lack of bone erosion along with the clinical aspects, this is probably a congenital type.
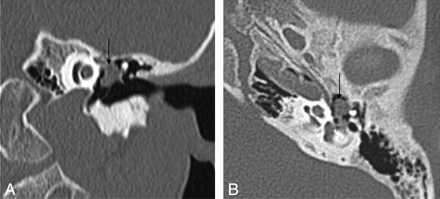
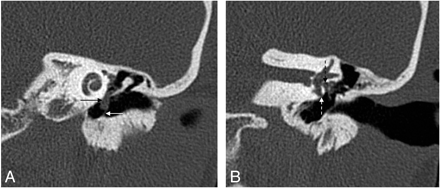
Cholesteatoma of the petrous apex. Contrast-enhanced HRCT scans with a soft-tissue window (A) and a bone window (B) show an oval well-delineated, nonenhancing lesion (white arrows) with erosion of the posterior wall of the pyramidal segment of the internal carotid artery (thin black arrow) and the anterior wall of the jugular bulb (thick black arrow). C, DWI demonstrates diffusion restriction in the lesion (white arrow), supporting the diagnosis of a cholesteatoma.
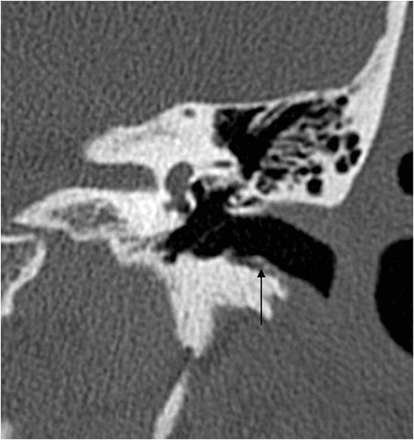
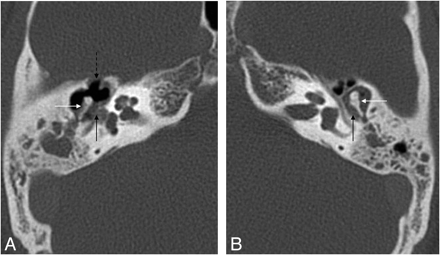
EAC cholesteatoma (arrow). Note the typical localization at the inferior wall of the EAC and the small bone fragments along the lesion.
“Primary acquired cholesteatomas” (80% of all middle ear cholesteatomas) develop behind an apparently intact TM, usually in the region of the pars flaccida.
“Secondary acquired cholesteatomas” (18% of all middle ear cholesteatomas) grow into the middle ear through a perforated TM, usually through the pars tensa and sometimes the pars flaccida.
Classification Based on Location in the Tympanic Cavity in Relation to the TM
On the basis of their site of origin, middle ear cholesteatomas (Table 2 can be classified as the following:
Classification of middle ear cholesteatomas based on location in relation to the TM
“Pars flaccida (attic) cholesteatomas” (Fig 4A) are located at the upper one-third portion of the TM (pars flaccida = Shrapnell membrane), filling the Prussak space (Fig 4B). On the basis of their pathogenesis, they are mostly acquired cholesteatomas resulting from a chronic infection with formation of granulation tissue behind an apparently intact TM (primary acquired) or through a perforation of the TM (secondary acquired).4 Congenital cholesteatomas may be present in this location as well.
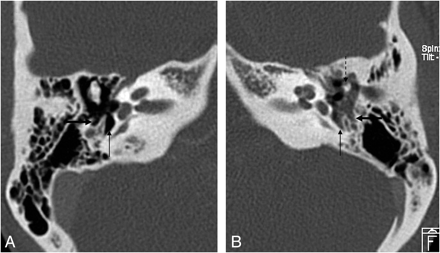
HRCT scan, coronal view. A, Pars flaccida cholesteatoma (arrow) filling the Prussak space. Notice the erosion of the scutum (dashed arrow). B, Prussak space is bordered by the pars flaccida of the TM (arrow) lateral, neck of the malleus (thick white arrow) medial, the short process of the malleus (white arrowhead) inferior, and lateral malleal ligament (dashed arrow) superior.
Initially, pars flaccida cholesteatomas are usually located lateral to the ossicles (Fig 4A).
“Pars tensa (sinus) cholesteatomas” develop most often through a defect of the lower two-thirds portion of the TM (pars tensa) (Fig 5) and most often are localized in the facial recess and sinus tympani of the tympanic cavity (Fig 6) and in the mastoid region.5 On the basis of their pathogenesis, they are either secondary acquired or congenital cholesteatomas.
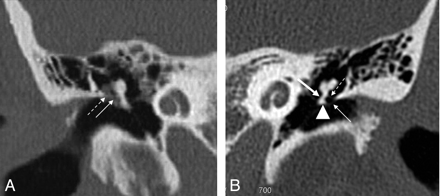
Pars tensa cholesteatoma. A, Coronal HRCT scan at the level of the cochlea shows the soft-tissue mass (black arrow) at the pars tensa of the retracted TM (white arrow). B, At the level of the vestibulum, the obliteration of the oval (dashed black arrow) and round (dashed white arrow) window niche is seen. Note the small bony fragments in the oval window niche, probably a sign of erosion of the stapes and the inferior wall of the tympanic segment of the facial canal.
Pars tensa cholesteatoma. HRCT scans demonstrate the normal (air-filled) (A) and the obliterated (B) sinus tympani (arrow) and facial recess (thick arrow) due to a pars tensa (sinus) cholesteatoma. Note the ossicular erosion (dashed arrow) on B.
Initially, pars tensa cholesteatomas are mostly located medial to the ossicular chain (Fig 5).
It is not possible to define the origin of an advanced lesion; hence neither of these classification subgroups is applicable.
Special Groups of Cholesteatomas
“Mural cholesteatomas” (Fig 7) are extensive lesions in the middle ear/mastoid, which drain their cystic contents through the TM into the EAC and leave the matrix behind. Due to enzymatic activity, the cavity grows continuously and resembles a mastoidectomy cave with no history of surgery; hence, the name of the process, “automastoidectomy.”6,7
“EAC cholesteatomas,” because of their particular age distribution (in older populations), peculiar etiology, and distinct clinical signs and differential diagnosis (see below), represent another special group of cholesteatomas. They are subdivided into idiopathic and secondary EACCs.8 The typical location of an idiopathic EACC is the floor of the EAC (Fig 3) with a characteristic bilateral occurrence. The location of secondary EACCs depends on the site of the inducing factor.
Mural cholesteatoma. Axial (A) and coronal (B) HRCT scans show the shell of the cholesteatoma in the epitympanum (black arrow) and the automastoidectomy cavity (thick arrow) without a history of surgery. Note the complete erosion of the ossicles, the fistula of the horizontal semicircular canal (dashed black arrow), and the wall erosion of the tympanic segment of the facial nerve canal (white arrow).
Histology
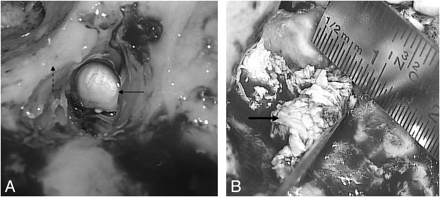
Cholesteatomas appear macroscopically as pearly gray or yellow well-circumscribed lesions (Fig 8A). However, if accompanied by granulation tissue, they present with a soft waxy material, discolored by inflammatory changes (Fig 8B).
Intraoperative images show a typical pearly appearance of a cholesteatoma (arrow, A), in the aditus ad antrum, next to the posterior wall of the EAC (dashed arrow) and a more irregular cholesteatoma (thick arrow, B) accompanied by granulation tissue.
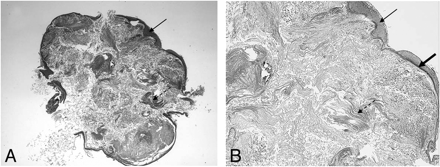
Histologically, cholesteatomas display a squamous cell cyst (Fig 9) representing an external matrix formed by a stratified strongly keratinizing squamous epithelium, which is similar to epidermal tissue elsewhere. The matrix produces abundant keratin lamellas, which are peeled off and pulled into the cyst.9 The matrix is usually accompanied by an adjacent external component containing collagenous and elastin fibers, mixed inflammatory cells, granulation tissue, and newly formed vessels called “perimatrix.” Bone fragments are often found within the perimatrix.
Histologic appearance of a cholesteatoma. Hematoxylin-eosin stain. Low-power view (original magnification ×25) (A) and high-power view (original magnification ×400) (B) demonstrate a cystic lesion covered by a strongly keratinizing stratified squamous epithelium (arrows). Within the cyst, there is abundant formation of desquamated keratin lamellas (dashed arrows). Note the prominent strongly hyperchromatic basal layer of the epidermis (thick arrow).
Some studies reported deoxyribonucleic acid aneuploidy in cholesteatomas, from which they postulated that they may be low-grade neoplasms.10 However, others found normal deoxyribonucleic acid distribution in cholesteatomas.11 Currently, the widely accepted opinion is that cholesteatomas are not neoplasms. Nevertheless, association between middle ear cholesteatoma and squamous cell carcinoma (ie, progression from cholesteatoma to squamous cell carcinoma) has been observed and reported in the literature.12
Bone erosion associated with cholesteatomas is reported in 80%–96% of cases, with a higher incidence in children than in adults and in pars flaccida than in pars tensa cholesteatomas.13–15 Congenital cholesteatomas cause ossicular erosion less frequently, to a lesser extent, and later in the course of the disease than acquired cholesteatomas.
Pathophysiology
Multiple theories were developed concerning the pathophysiology of both congenital and acquired cholesteatomas. Which one is the most plausible is a matter of long-standing debate and still remains unclear.16
In light of several otopathologic findings,17,18 the most plausible explanation for the development of congenital cholesteatomas is the postpartum persistence of a fetal epithelial thickening medial to the malleus neck. This lesion is a distinct squamous cell nest with unknown function, which usually involutes to become normal endothelium. Lack of involution, for unknown reasons, can be the precursor of congenital cholesteatoma,19 which may form within the uterus or early in life. Congenital cholesteatomas can be associated with EAC atresia or, rarely, with first branchial cleft remnants.
Acquired cholesteatomas may develop by various etiopathogenic mechanisms:
The “migration theory” postulates relocation of squamous epithelium from the margin of a perforated or retracted TM into the middle ear, forming a cholesteatoma.20
The “basal hyperplasia theory” assumes an inflammation-associated proliferation of basal cells breaking through the basement membrane, thus giving rise to a cholesteatoma.21
The “postsurgery/posttraumatic theory” claims iatrogenic implantation of epidermal elements into the middle ear cavity.22
According to the “retraction pocket theory,” the main trigger for cholesteatoma formation is poor ventilation of the mastoid-cave and the middle ear as a result of eustachian tube dysfunction. This leads to an increased negative pressure in the tympanic cavity, resulting in retraction of the TM with invagination of part of it, usually the pars flaccida. Chronic retraction pockets may facilitate hyperplastic epidermal in-growths into the middle ear with consecutive development of granulation tissue and bone erosion. On the basis of this mechanism, cleft palate23 and poorly pneumatized mastoids (underdeveloped or as a consequence of chronic inflammation)24 are associated with a higher risk for cholesteatoma formation. The developing cholesteatoma may cause secondary perforation in the TM. It is not known why some retraction pockets are transformed into cholesteatomas and others are not; an associated inflammation leading to hyperkeratinization has been suggested to play a role.9
The “metaplasia theory” is based on the identification of mucous glands in the inflamed connective tissue taken from cholestematous ears.25 However, various studies26,27 revealed that acquired cholesteatomas originate from the squamous epithelium (ectoderm) of the EAC and the bordering TM, rather than from metaplasia of the cuboidal epithelium (endoderm) of the middle ear.
For the development of idiopathic EACC, rudiments of the first branchial cleft or dissemination of the germinal epithelium might be responsible. Repeated microtrauma (cotton-tipped applicators, hearing aids) and diminished microcirculation (eg, from smoking) might be risk factors as well.8
Secondary EACCs occur at the site of idiopathic stenosis or narrowing of the EAC due to a lesion (such as an osteoma, exostosis, nevus, or mycetoma) or following trauma, surgery, inflammation, or radiation therapy.8
Clinical Signs
The clinical presentation of a congenital cholesteatoma may be an asymptomatic white mass behind an intact TM, usually discovered incidentally. Unless the cholesteatomas grow large and result in infection, these patients have no history of ear disease. In an advanced stage, however, congenital cholesteatomas cause the same symptoms as acquired ones (see below).
Patients with acquired cholesteatomas have a history of recurrent ear disease, eustachian tube obstruction, atelectasis of the middle ear, and reduced pneumatization of the mastoid. Primary acquired cholesteatomas are found behind an apparently intact TM, whereas secondary acquired cholesteatomas present with a perforated TM (most often on the pars tensa, sometimes on the pars flaccida).
According to several studies,28–32 middle ear cholesteatomas present with the following clinical signs:
Chronic discharge of the ear is present in 33%–67%, and some form of hearing loss, in 60%–87% of patients. Most patients present with mixed hearing loss, less frequently with sensorineural hearing loss or with a dead ear.
Facial nerve paralysis occurs rarely with middle ear cholesteatomas but can be present in 20%–64% of extensive cholesteatoma cases, half of which are higher grades.
Vertigo affects 30%–60% of the patients, whereas tinnitus, otalgia, and headache are less common manifestations.
Recurrent cholesteatomas after surgery are observed in 5%–13% of cases.
EAC cholesteatomas present with otorrhea, otalgia, and conductive hearing loss.8 Recurrences are more commonly seen in cases of larger lesions and of associated bony erosions.
Cross-Sectional Imaging
CT
HRCT is the imaging technique of choice in case of a clinically suspected cholesteatoma. HRCT, due to its excellent spatial resolution, has a high sensitivity with a high NPV when it shows a free middle ear or mastoid. However, specificity is low in the case of a mass lesion because it may correspond to granulation tissue, secretion, cholesterol granuloma, or neoplasm. Still, the location of the mass and the absence or presence of bony erosions give some hints to the pathology of the lesion. Typical findings associated with cholesteatoma include a sharply marginated expansile soft-tissue lesion, retraction of the TM (Fig 5A), scutum blunting (Fig 4A), and erosion of the tympanic tegmen and ossicles (Fig 10 A). Holotympanic absence of bony changes is suggestive of otitis media without cholesteatoma formation, whereas presence of bony erosions (along with clinical suspicion) indicates cholesteatoma (Fig 10).
Patient with cholesteatoma on the right and chronic otitis media without cholesteatoma on the left. A, Axial HRCT scan shows the mass lesion (black arrow) in the tympanic cavity with ossicle erosion (white arrow) and erosion of the anterior wall of the epitympanum (dashed arrow). B, Axial HRCT scan demonstrates a mass lesion (black arrow) in the epitympanum, but no bony erosion (white arrow).
However, there are some limitations concerning the pathognomonic value of bony erosions. Fifty to 97% of patients have CT evidence of the intraoperatively proved bony changes,33–35 depending on the location of the erosion. Moreover, the occurrence of erosions is variable in the different types of cholesteatomas (congenital cholesteatomas—rarely; pars flaccida cholesteatomas—in 70% of cases; pars tensa cholesteatomas—in 90% of cases). Therefore, the absence of bony destruction does not exclude cholesteatoma, especially if the lesion is small. Unfortunately, the typical contrast-uptake pattern of cholesteatomas (none or ringlike contrast enhancement) is not very visible on CT, especially if the lesion is small. However, in larger cholesteatomas, the absence of central contrast enhancement is a useful sign for the differential diagnosis (Fig 2A). The CT attenuation of cholesteatomas is not specific for differentiating them from the accompanying effusion or granulation tissue. Thus, CT is unable to accurately define the exact location and the extension of the lesion, especially in a temporal bone having already undergone surgery (Fig 11 A).
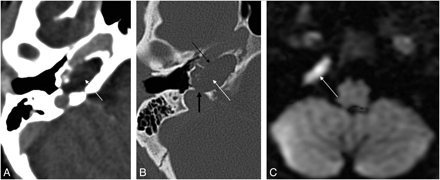
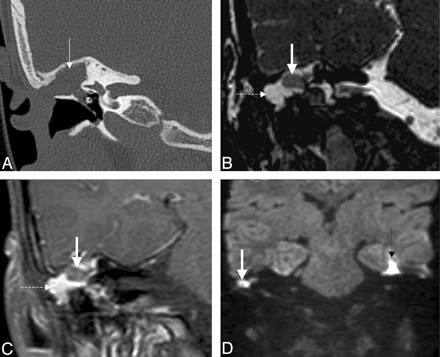
Recurrent cholesteatoma after surgery. A, Coronal HRCT scan shows the obliterated mastoidectomy cavity (white arrow). B, Coronal FIESTA image distinguishes the slightly hyperintense (to brain) cholesteatoma (thick white arrow on B, C, and D) from the strongly hyperintense granulation tissue (dashed arrow on B and C). C, Coronal contrast-enhanced MR image differentiates as well the nonenhancing cholesteatoma from the strongly enhancing granulation tissue. D, Coronal DWI with the intensive intralesional diffusion restriction supports the diagnosis. Note the susceptibility artifacts on the EPI-DWI (black arrow) on the right.
MR Imaging: Conventional Techniques
MR imaging provides complementary information due to the different pulse sequences, leading to a better tissue differentiation. The MR imaging signal-intensity characteristics of cholesteatomas are not specific, usually hypointense/isointense on T1WI and hyperintense on T2WI compared with brain tissue. Granulation or scar tissue in an ear that already underwent surgery and bloody serous or proteinaceous fluid also show hyperintense signal intensity on T2WI. Sometimes, cholesteatomas have lower signal intensity on T2WI or on constructive interference in steady state/FIESTA images than the surrounding granulation tissue (Fig 11B); however, they may also be indistinguishable on these sequences. On contrast-enhanced T1WI, differentiation may be possible,36 because granulation tissue shows contrast enhancement, whereas cholesteatoma does not (Fig 11C). However, in a temporal bone having already undergone surgery, standard MR imaging sequences may not accurately detect cholesteatomas.37
MR Imaging: Novel Techniques
In the past 7 years, several reports have shown that diffusion-weighted single-shot spin-echo echo-planar sequences can be useful in the diagnosis of cholesteatomas.38–40 Irrespective of their type (congenital or acquired), cholesteatomas appear to have high signal intensity on DWI (Fig 11D), attributed partly to restricted water diffusion (probably due to the oily consistency of the contained fluid) and predominantly to the T2 shinethrough effect of the lesion as revealed by calculated apparent diffusion coefficient values.41 In the study of Vercruysse et al,41 a high sensitivity of 81% and a specificity of 100% with a PPV of 100% and an NPV of 40% were achieved for detecting cholesteatomas in patients who had not undergone surgery. However, in a temporal bone that had undergone surgery, the sensitivity dropped to 12.5%, whereas the specificity remained 100%, with a PPV and NPV of 100% and 72%, respectively. The false-negative cases corresponded to cholesteatomas <5 mm and to mural cholesteatomas. In other studies on recurrent/residual cholesteatomas in an ear that had undergone surgery,40,42 the sensitivity of 77%–86% and the NPV of 75%–92% showed considerably better results.
The few false-positive cases with DWI described in the literature turned out to be acute otitis media,38 bone powder,43 scar tissue,44 a silastic sheet,45 granulation tissue,45 cholesterol granuloma,46 and endocrine adenoma (in our series, unpublished data, K.B., March 2007).
A limitation of spin-echo DWI in the detection of a residual/recurrent cholesteatoma is mainly attributed to the presence of magnetic susceptibility inhomogeneities at air/bone interfaces at the skull base,47 especially at the tympanic tegmen (Fig 11D). These artifacts theoretically can disguise a cholesteatoma, though in reality, they can be distinguished due to their rather linear pattern, being situated outside or at the ridge of the petrous bone, and due to their location, which does not correspond to the lesion detected on the other sequences and CT. Nevertheless, these artifacts can be reduced by parallel imaging techniques as well as multishot EPI and FLASH sequences.48
Ayache et al49 suggested delayed contrast-enhanced T1WI (30–45 minutes after contrast injection) for detection of recurrent cholesteatoma in case of complete opacity of the tympanomastoid cavity on CT. They achieved good diagnostic results (sensitivity, 90%; specificity, 100%; PPV, 100%; NPV, 92%), when the diameter of the cholesteatoma was at least 3 mm. Despite its clinical impracticality due to the prolonged examination time, this approach may be useful in selected cases for the diagnosis of recurrent cholesteatoma.
Venail et al45 compared DW-EPI with delayed contrast-enhanced MR imaging in patients having undergone surgery and found DWI to be more specific but less sensitive than delayed contrast-enhanced MR imaging. Specificity was further improved by using both techniques only for cholesteatomas >5 mm.
Some reports have discussed the value of a DW-non-EPI sequence in the diagnosis of cholesteatoma in nonoperated50,51 and operated temporal bone.43,52,53
Single-shot turbo spin-echo DWI allows the use of a higher imaging matrix and thinner (2 mm) sections and is associated with fewer susceptibility artifacts. This sequence allows detection, in an ear that has not undergone surgery, of a cholesteatoma as small as 2 mm.51 Sensitivity, specificity, PPV, and NPV of this technique in the diagnosis of postoperative residual cholesteatoma were 90%–100%, 100%, 100%, and 96%–100%, respectively, which make it possible to avoid unnecessary second-look operations.43,52
The most recent method for the diagnosis of recurrent cholesteatoma is the multishot fast spin-echo DWI-PROPELLER technique.
Lehmann et al54 compared DWI-PROPELLER with array spatial sensitivity encoding, single-shot, echo-planar DWI by using a 3T scanner. Due to fewer artifacts and better contrast, they found better detectability of recurrent cholesteatomas with the DWI-PROPELLER technique. The smallest lesion detected with this technique measured 3 mm. Its limitations, however, are that for the time being, it allows only axial sections to be obtained and is associated with prolonged acquisition times, which can lead to motion artifacts. Whether this technique provides better results than the DW-non-EPI sequence needs further comparison between the 2 sequences, also by using clinically more available 1.5T MR imaging scanners.
In summary, HRCT and MR imaging with DWI remain complementary examinations, which are both necessary in the accurate diagnosis of a recurrent/residual cholesteatoma to avoid unnecessary second-look operations.
Imaging of Complications
Labyrinthine fistula is the most frequent complication associated with middle ear cholesteatoma, with a prevalence of 5%–10%. Episodic vertigo, sensorineural hearing loss, tinnitus, and the CT finding of a dehiscent lateral semicircular canal (Fig 7B) support the diagnosis. A dehiscence on the cochlear promontory represents an uncommon location for labyrinthine fistula. A fistula of the oval window might be suspected on HRCT when a stapes fragment is dislocated toward the vestibulum. Facial palsy may result from direct inflammatory effects, compression atrophy, very rarely from the presence of an inflammatory neuroma,55 and most often from erosion of the tympanic segment of the facial canal (Fig 7B). However, bony dehiscence of the tympanic segment of the facial canal is a common variant present in 25%–57% of cases.56 Perineural extension of a cholesteatoma along the facial nerve may also occur,57 in which case MR imaging is important, to exclude a neoplasm.
Sensorineural hearing loss develops by cholesteatomatous involvement of the internal auditory canal.58 Total hearing loss may occur in the presence of a labyrinthine fistula causing labyrinthitis, which can be diagnosed by MR imaging showing enhancement of the membranous labyrinth. In these cases, CT is useful for demonstrating labyrinthine ossification ensuing in complicated or untreated cases. Erosion of the sigmoid sinus plate (Fig 2B) and consecutive thrombosis, tympanic tegmen erosion and subsequent intracranial invasion, recurrent bacterial meningitis, and intracranial abscess are rare complications, which, nevertheless, require an urgent MR imaging examination. A bony defect of the tympanic tegmen or the anterior wall of the epitympanum should raise the suspicion of an encephalocele or cholesteatoma extension in the middle cranial fossa, and MR imaging is recommended.
Differential Diagnosis
The spectrum of the differential diagnosis of cholesteatomas (with the exclusion of EACCs) includes the following pathologic entities:
“Cholesterol granulomas” are typically non-contrast-enhancing lesions and show high signal intensity on T1 and T2-weighted MR imaging. Bony erosion is usual. In an otoscopic examination, the TM is blue, and there is a history of surgery or recurrent otitis media. DWI does not show diffusion restriction, though 1 false-positive case of cholesterol granuloma was published.46
“Paragangliomas” appear otoscopically as pulsatile vascular masses behind the TM. CT and MR imaging show a nodular enhancing mass on the cochlear promontory without bony erosions.
“Schwannomas of the facial nerve and geniculate ganglion” present on CT with an enlarged facial nerve canal and geniculate fossa with a characteristic tubular/oval enhancing mass along the tympanic segment of the facial nerve on MR imaging.
“Facial nerve hemangioma” also presents on CT with an enlarged facial nerve canal and geniculate fossa. The ossifying type causes a “honeycomb” morphology, which helps in its differentiation from other masses of this region. On MR imaging, hemangiomas are slightly hypointense/isointense on T1WI and hyperintense on T2WI and appear as strongly contrast-enhancing oval lesions on postcontrast images, associated with irregular poorly defined margins.
The differential diagnosis of EAC cholesteatomas includes the following:
“Keratosis obturans” presents with bilateral keratin plugs within the enlarged EAC, usually in young patients with sinusitis and bronchiectasis. It can be in association with chronic otitis externa or previous surgery and can be easily diagnosed by clinical examination. Noncomplicated cases usually do not show bony erosion.
“Necrotizing external otitis” develops commonly in elderly immunocompromised patients (usually with diabetes) with Pseudomonas aeruginosa infection. Extensive bony erosions and strong contrast enhancement at the base of skull are seen.
“Squamous cell carcinoma” of the EAC is mostly seen in elderly patients and sometimes may be indistinguishable from a cholesteatoma.
Indicates open access to non-subscribers at www.ajnr.org
References
- Copyright © American Society of Neuroradiology