Abstract
BACKGROUND AND PURPOSE: Flow-diverter stents are an alternative treatment for challenging and recurrent aneurysms. Thrombosis of the sac is thought to induce perianeurysmal brain inflammation, but such phenomena have never been studied in flow-diverter devices. We developed imaging data to explain the clinical exacerbation of symptoms after flow-diversion treatment.
MATERIALS AND METHODS: Seventeen patients with unruptured aneurysms were treated by using a flow-diverter device. Clinical symptoms and angiographic and MR imaging features were recorded before and after treatment, during both the acute and chronic phases, to look for inflammatory reaction.
RESULTS: Seven of the 17 patients (41%) showed a delayed clinical aggravation of symptoms posttreatment consisting of a headache (n = 7) with aggravation of pre-existing compressive symptoms (n = 4) and the appearance of compressive symptoms (n = 1). This clinical deterioration was transient; it was observed between 3 and 15 days posttreatment and resolved by day 30. MR imaging revealed signs highly suggestive of perianeurysmal inflammation with vasogenic edema and blood-brain barrier breakdown. The association between MR imaging inflammatory features and clinical aggravation was statistically significant. Large aneurysmal size and its proximity to surrounding brain tissue were predictive of this inflammatory reaction after flow diversion.
CONCLUSIONS: The main finding of the series is that MR imaging−defined perianeurysmal inflammation is observed with a high frequency after treatment of unruptured aneurysms with flow diverters and is, in most cases, associated with a transient clinical deterioration.
Abbreviations
- IL1β
- interleukin 1-β
- NF
- nuclear factor
Flow-diverter stents are one alternative in the treatment of large and giant aneurysms, wide-neck aneurysms, and aneurysm recurrences. In these cases, embolization with coils is often associated with incomplete occlusion or early recurrence. A coil/stent combination improves long-term results, but it increases associated morbidity.1 A new stent prototype appeared in 2007 and consisted of a high-attenuation braided mesh stent aimed at redirecting flow from the aneurysm sac toward the parent artery.2,3 The goal of this diversion is to obtain a rapid flow reduction, which in turn leads to thrombosis of the aneurysm sac as demonstrated in animal experiments.4
Rapid thrombosis of a voluminous intracranial aneurysm was suspected to cause an endosaccular reaction, thus possibly increasing compression due to the aneurysm. This complication is thought to occur after clamping of the parent artery, as in the case of treatment for a carotid siphon giant aneurysm5 or after coil placement in the aneurysm sac.6 Nevertheless, this phenomenon and its clinical impact have never been studied with flow-diverter stents, to our knowledge, despite their ability to induce thrombosis in the aneurysm sac. Consequently, we prospectively followed 17 patients treated for intracranial aneurysms with a flow diverter to describe the clinical and MR imaging data suggestive of posttherapeutic perianeurysmal inflammation.
Patients and Methods
Study Population
Seventeen consecutive patients, 10 women and 7 men, ranging from 21 to 82 years of age (mean age, 48 years) were prospectively included from January 2009 to February 2010. Inclusion criteria were the following: 1) unruptured large intracranial aneurysms and 2) indication for flow-diverter stent placement (Silk; Balt Extrusion, Montmorency, France), either because of aneurysm recurrence after coil treatment or because of an ineligibility for conventional coil/stent treatment due to a large diameter and/or large neck width. Before the procedure, benefits and risks were explained and all patients were required to provide their informed consent. Specific information related to this MR imaging−based study was given to the patient and documented in the medical records. All the procedures had been practiced, and the products were used in the usual way. Authorization by relevant French authorities and ethics committee approval were not required by law for this noninterventional study.
Clinical, MR imaging, and 3D angiographic data were systematically collected before treatment. The following data were recorded from MR imaging and angiography: 1) the anatomic relationship between the aneurysm sac and the cerebral parenchyma (ie, was the aneurysm wall embedded in the brain or surrounded by CSF), 2) the presence of a partial pre-existing intrasaccular thrombosis, 3) the dimensions of the sac, and (4) the total surface of the aneurysm wall. This interface was measured by using the equation for a spheroid by taking into account both the small (a) and large (b) diameters of the aneurysm:
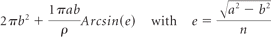
Treatment
Treatment by using a Silk stent was performed without additional coil placement in the sac to avoid an increase in the mass effect.
Double antiplatelet therapy was administered from 3 to 7 days before the procedure until 2–3 months after the procedure, combining clopidogrel, 75 mg per day, and aspirin, 160–250 mg per day. Then the combination was replaced by aspirin treatment alone for 1 year. A heparin bolus (50 IU/kg) was administered at the beginning of the procedure and was discontinued at the end. A 3-week steroid taper was administered to 4 patients the day of Silk stent implantation, and in 2 patients, the taper was administered after compressive symptoms had already appeared (On-line Table).
Follow-Up
The initial surveillance phase was tightly controlled and included a daily neurologic examination either by a neurologist, a neurosurgeon, or a neuroradiologist. After discharge, neurologic examinations were scheduled at 1 and 3 months. Aneurysm thrombosis after stent placement was evaluated by repeated vascular imaging. Systematic MR imaging was scheduled at 3 months and 1 year and arteriography, at 3 months. Long-term MR imaging was also repeated in some patients (n = 13, up to 12 months following treatment). Additionally, in patients presenting with clinical signs, MR imaging was performed between 3 and 15 days posttreatment. All the examinations were performed with an Achieva 1.5T magnet (Philips Healthcare, Best, the Netherlands) and included axial FLAIR, diffusion, MR angiography, and T1 postgadolinium sequences. The following criteria were analyzed on MR imaging: 1) rate of aneurysm thrombosis, 2) in-stent arterial diameter, 3) edematous reaction around the aneurysm sac on FLAIR and diffusion images, and 4) perianeurysmal enhancement.
Monitoring of angiographic evolution was done by using either MR angiography or angiographic data. The analyzed imaging criteria included the following: 1) the rate of aneurysm sac occlusion, 2) parent vessel permeability, or 3) any intrastent stenosis.
Statistics
Analyses were performed by using R software (Version 2.11.1, http://www.r-project.org/). We defined 2 groups of patients: namely, those with aggravated symptoms after treatment (worsened) and those without any worsening of their clinical status (unchanged). A comparison of variables between groups was performed by using the nonparametric Mann-Whitney U test or the Fisher exact test, when appropriate. Correlations between clinical status after treatment (worsened or unchanged) and imaging variables were calculated by using the Pearson test. A P value < .05 was considered significant.
Results
Before treatment, 7 of 17 patients were symptomatic, of whom 6 had a compression syndrome and the last had a postembolic right hemiparesis related to a dissecting and partially thrombosed left Sylvian fusiform aneurysm. Ten patients had a fusiform aneurysm, and the remaining 7 had a saccular aneurysm (Fig 1A, -B). The On-line Table summarizes the clinical and imaging data.
A, The angiogram shows a giant aneurysm that developed at a fenestration of the vertebrobasilar junction. B, A study of the left vertebral artery just after deployment of the flow diverter shows stagnation of the contrast media and complete thrombosis 3 months postprocedure. C and D, In the same patient, the baseline MR image shows a small area with high signal intensity on FLAIR-weighted imaging and a circulating aneurysm on T1-weighted imaging without perianeurysmal enhancement. In the same patient, MR imaging repeated 10 days after Silk-treatment, while clinical symptoms are worsening, shows a wide area with high signal intensity on the FLAIR image (E), circumferential aneurysmal wall enhancement after contrast media (F), and no ischemic signs on trace diffusion-weighted imaging (G). In the same patient, 9 months after treatment, there is no longer peri-aneurysm edema on the FLAIR sequence (H), and the aneurysm is totally excluded from the circulation on MR angiography (I). Note total aneurysmal occlusion (I).
Delayed clinical worsening within 2 weeks of the procedure occurred in 7 of 17 patients (41%), a criterion that was used to separate the population into 2 posttreatment groups; worsened and unchanged patients. Clinical aggravation in the 7 patients consisted of constant headaches homolateral to the treated aneurysm, secondary to the treatment and not present until then. Headaches were isolated symptoms in 2 patients and associated with compression syndrome in 5 patients: Two presented with compression of the cavernous sinus, 1 presented with compression of the optic nerve, and 2 presented with compression of the brain stem. Regarding the course of these symptoms, they appeared either after treatment (patient 1) or were already moderately present before Silk placement but with strong (and transient) worsening after treatment. This clinical worsening appeared after a mean delay of 8.6 ± 4.1 days posttreatment (range, 3–15 days) with a resolution in all cases by 30 days posttreatment, except for 1 patient who experienced a moderate right hemianesthesia that persisted for 3 months after brain stem compression (patient 3).
We first examined any imaging parameters capable of explaining the clinical exacerbation of symptoms after treatment. According to MR imaging examinations performed between 3 and 15 days, none of the patients had secondary hemorrhage. Aneurysm size did not significantly increase after treatment in either the worsened group (mean largest diameter, 18.8 mm before and 17.6 mm after stent placement, P = .79) or the unchanged group (mean largest diameter, 12.8 mm before and 15.2 mm after stent placement, P = .52). There were no cases of parent artery thrombosis, and no clear infarcted areas in the worsened group. An associated small perianeurysmal area with low ADC values around a compressive thrombosed daughter sac was only observed in 1 patient (patient 3), who also had the worst clinical condition posttreatment.
Nevertheless, we found imaging changes highly suggestive of a perianeurysmal brain inflammatory reaction in 5 of 7 patients with aggravated symptoms (On-line Table). This presented as perianeurysmal brain edema with high signal intensity on the FLAIR images, increased ADC values, and circumferential postcontrast enhancement (Fig 1C–G). For unchanged patients after stent placement, MR imaging examinations performed during the first week revealed no signs of inflammation with the exception of 1 patient (patient 10) who presented with isolated and mild perianeurysmal contrast enhancement. The association between MR imaging inflammatory features and clinical aggravation was statistically significant (P = .02, Fisher exact test; r = 0.73, P = .004, Pearson correlation).
Considering that perianeurysmal inflammatory reaction could be the main cause for patient worsening, we looked for data on the MR imaging results that could be predictive of such a reaction. Patients exhibiting MR imaging inflammatory features had larger aneurysms than patients without any inflammation based on MR imaging data (mean largest size, 22 mm versus 13.1 mm, P = .028; a mean interface, 1340 mm2 versus 427 mm2, P = .018, respectively). To illustrate, patients with aggravated symptoms, but without clear inflammation shown on the MR imaging results, had the smallest aneurysms among the worsened patients and no other explanation for their transient clinical changes. The anatomic setting could also be important due to the fact that 100% of the patients with a post-flow-diverter clinical aggravation had an aneurysm embedded in the parenchyma without the interposition of CSF, whereas only half of patients without symptoms had such a feature. The rate of posttreatment thrombosis was not statistically different between patients exhibiting or not MR imaging inflammatory reactions.
All worsened patients had, from the onset of symptoms, an occlusion of at least 50% of the aneurysm volume. The 2 patients (On-line Table, patients 3 and 6) with acute occlusion within the first week were those who also had the most serious edematous reactions. Symptoms always resolved within 30 days posttreatment, except for patient 3. Some patients (n = 15) were re-imaged later; the inflammatory MR imaging signs had disappeared at examinations performed 3 months after flow-diverter placement (Fig 1H, I).
Discussion
In the present article, we report 41% of patients with neurologic worsening after flow-diverter treatment for unruptured cerebral aneurysms. We found MR imaging evidence of perianeurysmal brain inflammation after the therapeutic thrombosis of the sac to be the main cause explaining clinical aggravation. Consequently, we may consider a perianeurysmal brain inflammation when encountering the association of a postprocedure headache with an increase in previous compressive signs, possibly associated with MR imaging signs of inflammation.
Inflammation of the aneurysm wall has already been documented,7 but to the best of our knowledge, this is the first study describing perianeurysmal brain inflammation linked to post-flow-diversion thrombosis by using clinical signs and MR imaging findings. This phenomenon seems to exist with every endovascular technique that induces a rapid thrombosis of the aneurysm sac. Related observations have been reported in the literature after carotid occlusion for a carotid cavernous aneurysm.5 With coils, this inflammatory reaction occurs in 18% of cases6 and is documented by MR imaging, but it often remains asymptomatic. With HydroCoils (MicroVention Terumo, Aliso Viejo, California),6 this symptomatology has become more frequent. This complication has not been described after microsurgical treatment of an aneurysm, probably because most neurosurgeons puncture the aneurysm sac after clipping to shrink the sac, thus limiting the extent of thrombosis.
In 7 patients with worsening symptoms after Silk stent placement, we first looked for an ischemic or compressive cause. We did not find any significant increase of the aneurysm sac after treatment. Furthermore, permeability of the parent vessel was confirmed in all cases. Low ADC values on diffusion-weighted images were encountered in 1 patient, in contact with a compressive lobulation; this occurred in the only patient whose aneurysm occlusion was complete and immediate after the stent was deployed, thus resulting in rapid thrombosis of the aneurysm sac. MR imaging examinations performed before 15 days posttreatment in worsened patients identified a thrombosis of at least 50% of the aneurysm volume associated with brain vasogenic edema, which was revealed as a FLAIR hyper-signal intensity and perianeurysmal enhancement. This inflammation was not found on the pre-embolization MR imaging results. It is possible that the sac thrombosis activates an inflammatory reaction that spreads transparietally to the adjacent cortex and cranial nerves. Supporting this hypothesis, inflammatory reactions were found around aneurysms in close proximity to the parenchyma. A FLAIR hyper-signal intensity was less visible in worsened patients after treatment of a carotid cavernous aneurysm, probably because the dura mater of the cavernous sinus limits extension of the inflammation to the temporal lobe. Large aneurysmal size was the most significant parameter associated with an inflammatory reaction. Given that the total wall surface represents the interface between the thrombus and the surrounding parenchyma, it makes sense that inflammation spreading to adjacent tissue might be facilitated in giant aneurysms. The weakening role of the thrombus is well-known in abdominal aortic aneurysms8 and ruptured intracranial aneurysms.9
Our first hypothesis was an increase in aneurysm size during its thrombosis, but no modification of the aneurysm volume was noticed. A nonreturn valve mechanism at the level of the stent mesh has been proposed.10 The work of Seong et al4 did not support this type of mechanism because they demonstrated a reduction in circulatory speed within the aneurysm without an associated increase in intrasaccular pressures after stent placement in an animal model. In contrast, a drop in intra-aneurysm pressure can induce a phenotype modification of the endothelial cells that could participate in the genesis of local inflammation, as has been demonstrated in the carotid bulb.11
Recently, Stutz et al12 showed that the death of endothelial cells lining the aneurysm wall was associated with massive thrombosis, leading to perilesional cortical inflammation. Thrombosis of the aneurysm sac compromises its endothelium vascularization, resulting in anoxia/ischemia of the endothelium wall, cell death, and the release of stress signals collectively called the “damage-associated molecular patterns.” These signals are strong activators of inflammasome, which is a complex intracytoplasmic protein assembly leading to the activation of caspase 1. This in turn serves as the starting point for the massive production of IL1β in surrounding tissue. The cellular signaling of IL1β proceeds via nuclear translocation of a key inflammation transcription factor, namely, NF-κ B. According to this scheme, steroids, with the principal action of preventing this translocation, could be effective in inhibiting the effects of a massive release of IL1β. Furthermore, the amplitude of the edematous phenomenon should be proportional to the intensity of cell death at the internal surface of the aneurysm sac. The second phase of this reaction after the production of IL1β consists of local phagocyte recruitment.
Different degrees of severity may exist according to the importance of the enzymatic and macrophagic processes, from normal healing (asymptomatic, as shown with bare coils6) to more severe reactions (ie, MR imaging−depicted vasogenic edema reflecting diffusion of the process through the blood-brain barrier, as in our series). The final phase could be an inflammatory-mediated rupture that could explain delayed posttreatment bleeding with no obvious recanalization of the aneurysm sac.10
A similar poststenting inflammatory syndrome may also occur after endovascular repair of aortic aneurysms,13 which have a similar pathophysiologic hypothesis. Note that 3T MR imaging of aneurysm wall inflammation is possible14 by using a rabbit model, which in turn could help test these pathophysiologic hypotheses.
For postimplantation syndrome after endovascular repair of aortic aneurysms, steroids are considered as the first-line treatment.13 In our study, steroid treatment was given the day of treatment in 4 patients, but this treatment failed to prevent an inflammatory perianeurysmal reaction in 3 of these patients with the largest aneurysms. It is difficult to know whether we decreased this immunologic cascade in such large aneurysms. In 2 patients, steroid therapy was given after the onset of an inflammatory syndrome 5–6 days posttreatment. In both cases, headaches disappeared after 20 days. Thus, we are not able to determine if steroids modified the course of the headaches in such a small population and in the absence of a control group. For these 6 patients, the timing of treatment can be discussed because it was never given preventively. It would make sense to inhibit the origin of the inflammatory cascade on the basis of nuclear translocation of NF-κB. Alternatively, blocking IL1β binding with its cognate receptor IL-1RA (anakinra) might be considered as well.
Limits
During the immediate posttreatment period, we could not establish a relationship between aneurysmal occlusion and the appearance of inflammatory symptoms because of an insufficient number of MR imaging examinations. Three months after flow-diverter placement, we stated that the MR imaging inflammatory signs had disappeared, but we lacked the MR images necessary to establish a correlation between the regression of clinical signs and inflammatory images. Further studies are needed to optimize the timing of steroid treatment. Stent design and/or the introduction of coils into the aneurysm sac could be future research techniques used in animal models and human trials.
Conclusions
An inflammatory reaction may aggravate, transiently, clinical symptoms after aneurysm treatment with a flow-diverter device. A large-sized aneurysm and close contact with adjacent parenchyma were risk factors associated with perianeurysmal brain inflammation. Further research is needed to better understand the underlying mechanisms as well as to achieve better prevention strategies. These results may help patients avoid symptomatic aggravation during the first weeks after treatment.
Indicates open access to non-subscribers at www.ajnr.org
References
- Received January 15, 2011.
- Accepted after revision February 28, 2011.
- © 2011 by American Journal of Neuroradiology








