Abstract
BACKGROUND AND PURPOSE: ICAD with hemodynamic insufficiency may present with either fulminant infarct or with progressive neurologic deterioration. The purpose of this study was to evaluate the safety and efficacy of emergent self-expanding stent placement for acute intracranial or extracranial ICAD with significant hemodynamic insufficiency.
MATERIALS AND METHODS: Eight patients (7 men and 1 woman; age range, 20–55 years; NIHSS score, 5–21) underwent emergent self-expanding stent placement for treatment of significant hemodynamic insufficiency due to acute ICAD. The safety and efficacy of emergent self-expanding stent placement were retrospectively evaluated.
RESULTS: All patients presented with progressive (n = 6) or fluctuating (n = 2) neurologic deficits and revealed markedly decreased perfusion on CT or MR perfusion studies. Conventional angiography revealed acute occlusion (n = 2) or critical stenosis (n = 6) in intracranial (n = 3) or extracranial (n = 5) carotid arteries with a lack of sufficient collaterals. Stent placement was successful in all patients without any procedure-related complications. In all patients, hemodynamic insufficiency was corrected immediately after stent placement, and neurologic symptoms were completely resolved during several days. Mean improvement of the NIHSS score between baseline and discharge was 11.6 (range, 5–21). All patients remained neurologically intact (mRS, 0) during clinical follow-up for a mean of 21 months (range, 8–50 months). Angiographic follow-up was available for 6 patients at 3–12 months. None of the 6 patients revealed residual or in-stent restenosis.
CONCLUSIONS: Self-expanding stent placement is a safe and effective option for selected patients with significant hemodynamic insufficiency due to acute intracranial or extracranial ICAD.
Abbreviations
- ICA
- internal carotid artery
- ICAD
- ICA dissection
- mRS
- modified Rankin Scale
- NIHSS
- National Institutes of Health Stroke Scale
Although carotid and vertebral artery dissections occur in a minority of patients with stroke, such dissections are relatively common in young patients with stroke.1–3 The most common complication of ICAD is embolization of thrombi that develop on the intimal flap or in the pseudolumen.3 The natural history of ICAD may follow a benign course with conservative management. Another mechanism of stroke is hemodynamic insufficiency due to acute occlusion or critical stenosis of the true lumen due to dissection with the lack of sufficient collateral supply.4 In such cases, patients with ICAD present with either fulminant infarcts at initial presentation or with progressive neurologic deterioration. Therefore, early or emergent intervention may be required to treat cases of ICAD with significant hemodynamic insufficiency. In this study, we evaluated the safety and efficacy of emergent self-expanding stent placement for cases of intracranial or extracranial ICAD presenting with progressive or fluctuating neurologic deficits due to significant hemodynamic insufficiency.
Materials and Methods
The institutional review board approved this retrospective study. Informed consent was not required but was obtained for pharmacologic or mechanical thrombolytic treatment, including angioplasty and/or stent placement, from the patients' legal representatives. Eight patients underwent emergent self-expanding stent placement for treatment of acute intracranial or extracranial ICAD in 3 territory referral hospitals between April 2005 and November 2008. The patients included 7 men and 1 woman, with a mean age of 42 years (range, 20–55 years). The causes of ICAD were traumatic in 4 patients and spontaneous in 4 patients. The indications for stent placement were intracranial or extracranial ICADs causing progressive or fluctuating neurologic deficits in the presence of significant hemodynamic insufficiency on CT/MR perfusion studies and occlusion or critical stenosis with the lack of sufficient collateral supply on conventional angiography.
Intervention
Routine 3- or 4-vessel cerebral angiography was performed before treatment. After identification of the location of the ICAD causing acute occlusion or critical stenosis in the absence of sufficient collaterals, a guiding catheter (6F Envoy, Cordis, Miami Lakes, Florida; or 6F Shuttle-SL guide sheath, Cook, Bloomington, Indiana) was placed at the proximal portion of the relevant carotid artery. Placement of a self-expanding stent (Enterprise or Precise, Cordis; Neuroform or Wallstent, Boston Scientific, Natick, Massachusetts) was the initial mechanical maneuver for restoration of normal blood flow. The stent diameter was determined according to the diameter of the normal carotid artery proximal to the dissection, and the length of the stent was chosen to sufficiently cover the affected arterial segment. Any available stent of appropriate diameter and length to match the dissected ICA was used.
Embolic protection devices (FilterWire EZ, Boston Scientific or AngioGuard, Cordis) were used in 3 of 5 cervical ICAD stent-placement procedures. The use and choice of type of embolic protection device were left to the operator's discretion. Poststenting balloon angioplasty was performed in 3 of 5 cervical ICAD stent-placement procedures, in which cases >50% residual stenoses remained after stent placement. For intracranial ICA dissection, embolic protection devices and poststenting balloon angioplasty were not used due to both the technical difficulty and concern about potential risk of arterial injury. Immediately after stent deployment, a bolus of glycoprotein IIb/IIIa antagonist (tirofiban, 0.5–1 mg) was intra-arterially loaded in all patients due to lack of antiplatelet premedication. Anticoagulation was stopped, but intravenous infusion of glycoprotein IIb/IIIa antagonist (tirofiban) was maintained for 24–36 hours after completion of the procedure. A loading dose of dual antiplatelet medication (aspirin, 100–500 mg, plus clopidogrel, 300 mg) was administered immediately after the procedure. Maintenance doses of dual antiplatelet medication were administered for 3–6 months and then changed to aspirin monotherapy indefinitely.
Results
The Table summarizes the clinical characteristics, treatments, and outcomes of all 8 patients included in this study. All patients presented with progressive (n = 6) or fluctuating (n = 2) neurologic deficits. CT or MR perfusion was performed in all patients, all of whom revealed markedly decreased perfusion in the relevant brain parenchyma (Fig 1A, -B). The mean NIHSS score at treatment was 11.7 (range, 5–21). Conventional angiography revealed acute occlusion (n = 2) or critical stenosis (n = 6) at the intracranial (n = 3, Fig 2) or extracranial (n = 5) ICA along with a lack of sufficient collateral supply. Stent placement was successful in all patients without any procedure-related complications. In all patients, hemodynamic insufficiency was corrected immediately after stent placement (Figs 1 and 2), and presenting neurologic symptoms were completely resolved during several days. The NIHSS score at discharge was 0 in all patients except for 1 who had an NIHSS score of 1. The mean improvement of the NIHSS score between baseline and discharge was 11.6 (range, 5–21). All patients remained neurologically intact (mRS score, 0) during clinical follow-up for a mean of 21 months (range, 8–50 months). Angiographic follow-up was available in 6 patients at 3–12 months. None of these 6 patients had residual or in-stent restenosis.
Summary of clinical characteristics, treatment, and outcomes for 8 patients presenting with significant hemodynamic insufficiency due to ICA dissection
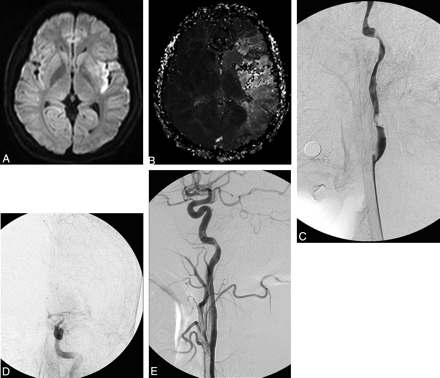
A 35-year-old man with a spontaneous left cervical ICA dissection. A and B, Diffusion- (A) and perfusion-weighted (B) images reveal a large area of diffusion-perfusion mismatch, suggestive of poor collateral circulation. C and D, Left common carotid angiograms reveal severe focal stenosis of the proximal left ICA due to dissection and also show poor visualization of intracranial arteries. E, Angiogram immediately after self-expanding stent placement reveals complete restoration of normal luminal size of the left ICA and well-visualized intracranial arteries.
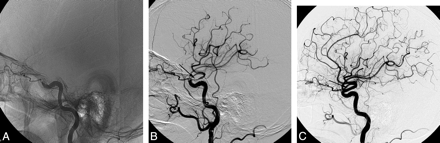
A 55-year-old man with a traumatic left intracranial ICA dissection. A, Left internal carotid angiogram reveals tapered occlusion of the left ICA just distal to the origin of the ophthalmic artery. Facial CT scan showed a linear fracture of the left optic strut adjacent to the left carotid artery (not shown). B, Lateral angiogram immediately after self-expanding stent placement shows recanalization of the ICA. Note incomplete expansion of the stent due to the remaining intramural hematoma (arrow). C, Follow-up lateral angiogram at 2 weeks reveals restoration of normal luminal size of the left ICA. Note complete resolution of the intramural hematoma and full expansion of the stent (arrow).
Discussion
ICAD is an important cause of stroke in young or middle-aged patients.1–3 Clinical manifestations of acute ICAD vary, with local symptoms, cerebral ischemia, or both.3 In acute ICAD presenting with ischemic symptoms, most strokes or transient ischemic attacks are embolic in nature; therefore, they are mostly associated with good outcomes after administration of anticoagulation and/or antiplatelet medications.5–12 Although stent placement for ICAD is both feasible and safe,13 it is reserved for selected patients with ICAD with recurrent embolism refractory to medication, with contraindications for anticoagulation, or with persistent/enlarging pseudoaneurysms.14–17 Stent placement for ICADs to reverse acute hemodynamic insufficiency or impending infarcts is described in only a few studies.18,19 Hemodynamic insufficiency due to acute obstruction or critical stenosis caused by dissection plays a key role in stroke progression in only a small number of patients with acute ICAD when combined with a lack of sufficient collateral circulation. In such cases, the patients have complete stroke at initial presentation or present with progressive neurologic deficits.4
Kremer et al20 reported a benign clinical course of unilateral ICAD causing severe stenosis or occlusion in 161 patients. However, in that study, 4 patients with permanent and 5 patients with transient severe stenosis or occlusion were excluded because they had undergone either bypass surgery or endovascular therapy for ICAD. Those 9 patients may be similar to those included in this study. Therefore, in the study of Kremer et al, approximately 5.6% (9/161) of patients with acute ICAD required surgical or endovascular intervention at initial presentation. Patients who survive severely stenotic or occlusive ICAD without stroke or progressive neurologic deficits at initial presentation may have sufficient collateral circulation to relevant brain tissue.
Because most severe stenoses and some occlusions eventually recanalize within weeks or months, embolic phenomena may be the only major complications in such patients.20,21 Therefore, conservative management with anticoagulation or antiplatelet medication may be sufficient, and further intervention may not be required in such patients. However, patients with impending infarcts or progressive neurologic deficits due to significant hemodynamic insufficiency may require early or emergent surgical or endovascular intervention.4,18,19
In this study, all patients presented with progressive (n = 6) or fluctuating (n = 2) neurologic deficits and revealed markedly decreased perfusion on CT or MR perfusion studies. Catheter angiograms also showed acute obstruction (n = 2) or critical stenosis (n = 6) with a lack of sufficient collateral supply. On the basis of the progression of neurologic deficits and radiographic findings, all patients underwent emergent self-expanding stent placement for correction of acute hemodynamic insufficiency due to intracranial (n = 3) and extracranial (n = 5) ICADs. All patients experienced excellent clinical and angiographic outcomes, with no procedure-related complications.
To the best of our knowledge, only 1 case of intracranial stent placement used to treat intracranially extended cervical ICAD as a part of multiple stent placement has been described in the literature.22 In that report, a self-expanding Neuroform stent was deployed from the intradural supraclinoid to the cavernous segment of the dissection, followed by tandem stent placement of 4 balloon-expandable stents covering the extradural-cavernous-to-cervical segments. Concerning the use of self-expanding stents to treat dissection, Ansari et al23 reported that the use of self-expanding Neuroform stents was successful for the treatment of 2 cervical ICADs and 7 high cervical or intracranial vertebrobasilar dissections.
In the current study, self-expanding stent placement was used safely and effectively for the treatment of intracranial ICADs and extracranial ICADs. It was also used in 2 obstructive and 6 stenotic ICADs. Our results correspond well with those of previous case series showing that stent placement is safe and effective for acute extracranial ICAD.14–19 Our results also suggest that this treatment may be safe and effective for intracranial acute ICAD. When stent placement is tried for treating an intradural dissection, one can never be too careful in navigating a guidewire to cross the dissection. Because the intradural arteries have little external elastic lamina, procedural rupture or aggravation of dissection may occur more easily in intradural arterial segments than in the extradural ones. Therefore, the guidewire should be withdrawn and renavigated if any resistance is felt during navigation. Fortunately, in all intradural ICA dissections in this series, the guidewire was easily negotiated through the dissected segment at the initial attempt. However, when difficulty is encountered in passing a guidewire through the dissected segment with any cause, an alternative surgical option, such as bypass surgery, should be considered.
The long-term patency of stented ICAs is a concern. However, in this study, all 6 patients for whom follow-up angiography was available revealed no residual or in-stent stenosis. This result corresponds well with other results reported in the literature.13,24 Therefore, delayed in-stent restenosis may not be a significant problem when treating ICA dissection by using stent-placement procedures.
Conclusions
Although most cases of ICAD have a good prognosis with conservative management, a small portion of ICAD cases with hemodynamic insufficiency due to poor collateral circulation may require immediate intervention. Self-expanding stent placement seems to be a safe and effective option for selected patients with significant hemodynamic insufficiency due to intracranial and extracranial ICAD.
Footnotes
-
Indicates Editor's Choices selection
-

-
This work was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare and Family Affairs, Republic of Korea (No. A085136).
Indicates open access to non-subscribers at www.ajnr.org
References
- Received January 31, 2010.
- Accepted after revision March 12, 2010.
- Copyright © American Society of Neuroradiology