Abstract
SUMMARY: Deep brain stimulation (DBS) is a new neurosurgical method principally used for the treatment of Parkinson disease (PD). Many new applications of DBS are under development, including the treatment of intractable psychiatric diseases. Brain imaging is used for the selection of patients for DBS, to localize the target nucleus, to detect complications, and to evaluate the final electrode contact position. In patients with implanted DBS systems, there is a risk of electrode heating when MR imaging is performed. This contraindicates MR imaging unless specific precautions are taken. Involvement of neuroradiologists in DBS procedures is essential to optimize presurgical evaluation, targeting, and postoperative anatomic results. The precision of the neuroradiologic correlation with anatomic data and clinical outcomes in DBS promises to yield significant basic science and clinical advances in the future.
Chronic high-frequency stimulation of the ventral intermediate nucleus (VIM) of the thalamus was first described in the early 1990s by Benabid et al.1 These authors implanted chronic stimulating electrodes in the VIM connected to a subcutaneous pulse generator positioned in the thoracic region to treat disabling tremor in 26 patients with Parkinson disease (PD) and in 6 with essential tremor.1 They demonstrated the effectiveness of this technique and its ability to produce complete relief from tremor. Improvement was maintained for up to 29 months. This was the first clinical demonstration that chronic high-frequency stimulation of nuclei (deep brain stimulation [DBS]) could replace destructive lesion-producing functional neurosurgery such as thalamotomy. This new technique was reversible and led to a renaissance in functional neurosurgery.
Next, the same team introduced bilateral DBS of the subthalamic nucleus (STN) in patients with disabling akinetic-rigid PD and severe motor fluctuations.2 This work extended data obtained in a 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine monkey model of PD showing that lesions and electric stimulation of the subthalamic nucleus reduced all of the major motor disturbances in this animal model. The results in patients with advanced PD were striking and were reproduced in many centers internationally. The US Food and Drug Administration approved the use of DBS for treatment of advanced PD with bilateral STN stimulation in 2002 and internal globus pallidus (GPi) stimulation in 2003. The main indication for DBS remains advanced PD, but numerous additional applications have been developed, ranging from dystonia to cluster headache, Tourette syndrome, and even psychiatric indications like obsessive-compulsive disorders (OCD) and major depression.
DBS is a neurosurgical method, but the role of neuroimaging in successful DBS intervention is critical. Neuroimaging is used for the preoperative selection of patients who will have DBS and to localize the intended target nuclei. In the postoperative period, imaging detects complications that uncommonly accompany the procedure, confirms the position of electrode contacts, and helps explain intended or unexpected effects. The involvement of neuroradiologists in DBS is mandatory to achieve excellent clinical results consistently. We will first describe the role of neuroimaging before electrode implantation, then its role for targeting, and finally its utility in the postoperative evaluation of patients.
Imaging of Patients before Electrode Implantation
The most common clinical indication for DBS worldwide is treatment of advanced PD. For this reason, the literature is most definitive on the preoperative evaluation of this patient population with brain imaging. Preoperative brain imaging (usually MR imaging) is used principally for the selection of those patients with PD who are candidates for DBS intervention (bilateral GPi or STN DBS). In most cases, the presence of abnormalities on MR imaging such as severe atrophy, leukoencephalopathy, or multiple lacunae contraindicates DBS surgery.3–5
MR imaging factors that predict good results of STN stimulation have been described.6 In patients who are candidates for bilateral STN stimulation, brain atrophy was not correlated with bad postoperative results. A normalized surface measure at a standardized level of the mesencephalon correlated with clinical effects of STN stimulation on motor disability in PD. A smaller mesencephalon surface area was associated with a decreased clinical benefit of stimulation. These MR imaging results are in accordance with clinical factors predictive of good postoperative outcome.5 The best hypothesis for explaining the clinical and imaging findings links levodopa-responsive symptoms that are caused by selective dopaminergic deficits with a preservation of mesencephalic-surface-area measurement and a good clinical response to STN stimulation. Conversely, a small mesencephalic surface area correlates with nondopaminergic non-levodopa responsive axial motor symptoms and cognitive impairment that are poorly responsive to STN stimulation.
Targeting
Indications for DBS and Target Nuclei
VIM Nucleus of the Thalamus.
Historically, VIM was one of the first nuclei targeted for DBS.1 The indication was PD and essential tremor.7,8 Today, VIM is still targeted for essential tremor, but the use of VIM DBS for PD is less frequent because of the recognition of other more effective target nuclei, STN and GPi, which participate in the 3 principal symptoms of PD (akinesia, hypertonia, and tremor) and not just tremor alone. VIM DBS has also been reported to relieve orthostatic tremor.9
Other Thalamic Nuclei.
Bilateral thalamic DBS has been used in Tourette syndrome.10,11 Very exciting results have been obtained in patients with a minimally conscious state, a disorder in the spectrum of persistent vegetative state. This suggests that DBS of certain midline thalamic nuclei like the central lateral nucleus, paralaminar regions of the median dorsalis, and the posterior-medial aspect of the centromedian/parafascicularis nucleus complex could produce arousal of the patient in a minimally conscious state. This work remains preliminary.12,13
Subthalamic Nucleus.
Today, most DBS interventions are bilateral implantation of electrodes within the STN5,14–17 for the treatment of advanced PD (Fig 1). This is by far the most carefully validated use of DBS, and the STN is the most thoroughly validated target nucleus. DBS of the STN has also been described in other indications like intractable epilepsy.18 Some case reports and a recent crossover double-blind multicenter study suggest that STN DBS may be effective in OCD.19–21
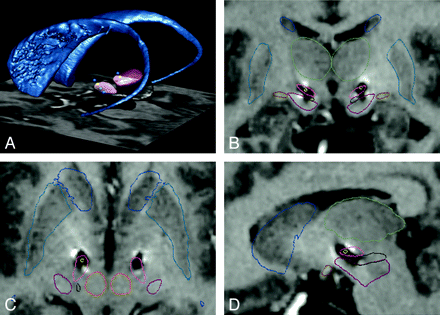
3D view of a postoperative MR acquisition in a patient with bilateral implantation of electrodes in the STN for the treatment of advanced PD. Caudate nuclei (blue), subthalamic nuclei (pink), electrodes (gray), and electrode contacts (blue) are segmented by using a 3D atlas described in Yelnik et al.55. A, Anterior oblique view. B, Posterior oblique view. C, Zoom on the electrodes showing that their contacts are located inside the STN.
GPi.
The main indications for GPi DBS are advanced PD4,22,23 and dystonia.24-29 GPi DBS has also been used to treat writer's cramp,30 and GPi is also targeted for the treatment of Tourette syndrome.31,32 In this indication, the effect of GPi DBS has been compared with the effect of centromedian-parafascicular complex (CM-Pf) of the thalamus DBS.31 It has been shown that GPi stimulation resulted in a dramatic improvement on the Yale Global Tic Severity Scale with a tic-severity reduction of 65%–96%. Bilateral stimulation of the CM-Pf was less effective with a reduction in tic severity from 30% to 64%.
Chronic Pain.
The treatment of intractable pain is one of the oldest indications for DBS. Several different targets have been used, among them periventricular/periaqueductal gray matter, the internal capsule, and the sensory thalamus.33,34
Miscellaneous.
Other applications of DBS have been presented in case reports or small series. The use of DBS for psychiatric indications like OCD20,35–37 or major depression38–42 has been described by using various targets. Hypothalamic DBS has been reported in cluster headache.43 Medically intractable seizures have been treated by using DBS of the anterior or centromedian nucleus of the thalamus;44 of the cerebellum;45 and of other targets like the STN, the hippocampus, and neocortical seizure foci.18
Targeting Techniques
Placement of electrodes for DBS is a difficult neurosurgical procedure that demands a high degree of precision. Accurate positioning of electrodes is mandatory to obtain optimal results. Most centers use the same 2-step procedure: First, the target location is determined using anatomic landmarks identified on MR images. The target is defined by using stereotactic imaging techniques, which allow determination of stereotactic coordinates relative to the stereotactic frame positioned on the patient's head. Next, the stereotactic target is confirmed and modified, if necessary intraoperatively, by using both microelectrode recordings and macrostimulation. Previously, invasive ventriculography was used to calculate the anatomic target for STN implantation, but this method is used uncommonly at present.46 MR imaging can safely be used for stereotactic targeting in DBS surgery, and it does not negatively affect the accuracy of the electrode implantation.47 It is necessary to understand the distortions that are produced in each specific MR imaging unit to use the stereotactic coordinates clinically.48 With proper quality assurance, it has been shown that it is possible to obtain excellent precision with MR imaging stereotactic data.48
Instead of using direct targeting with MR imaging in stereotactic conditions, it is possible to use MR imaging/CT fusion for anatomic localization.49 With this technique, CT is performed by using stereotactic techniques while the stereotactic coordinates and the outlines of the targeted nucleus are obtained with nonstereotactic MR imaging, and then the 2 datasets are fused.
Some reported the use frameless stereotaxy,50 with a skull-mounted trajectory guide and an image-guided workstation for DBS surgery. This technique, however, still requires surgical fixation of a device to the patient's head. Other authors compared the precision achieved with frameless neuronavigation and conventional frame-based stereotaxy.51 In this study, frameless neuronavigation was used on 1 side, and the frame-based technique, on the other side in the same patient. The authors compared the final electrode position with the planned position on the basis of intraoperative stereotactic plain x-ray. Electrode deviations from the target were larger using the frameless technique with a vector deviation of 2.5 mm than with the frame-based technique (vector deviation of 1.2 mm). In another small series of patients,52 real-time high-field interventional MR imaging has been used to implant electrodes for DBS. Other authors used open 0.2T operative MR imaging to perform DBS implants in 54 patients.53 A system consisting of a deformable computerized atlas of optimal target points, an electrophysiologic atlas, and an intraoperative graphic interface has been developed,54 allowing preoperative selection of target points and intraoperative optimization of the targets.
The development of a 3D histologic and deformable atlas of the human basal ganglia has also been described.55 MR imaging data were used for the coregistration of the atlas data. This permitted the production of anatomically and geometrically consistent 3D surfaces, by means of multimodal integration of Nissl calbindin cryosection photographic images, T1 and T2 MR imaging, and 3D contour optimization.
Practical Determination of Target Coordinates
Schematically, there are 3 different ways of determining the stereotactic coordinates of the target nuclei: 1) Coordinates can be statistically determined in reference to the anterior and posterior commissures; the statistical coordinates can be obtained from stereotactic atlases and/or from the experience of other groups.56 2) The target nucleus can be directly visualized on MR imaging, and finally, 3) it is possible to fuse MR imaging or CT data of the patient with a stereotactic atlas. The optimal strategy in a given circumstance depends on the location and MR imaging visibility of the target nucleus. For any given target nucleus, different strategies can be used successfully by means of 1 or combining 2 and even the 3 methods described above, depending on the experience of the interdisciplinary team.
STN Targeting.
Reported coordinates of the STN target are 12 mm lateral, 3 mm posterior, and 3 mm inferior to the midcommissural point;57 9–12 mm lateral, 1–2 mm posterior, and 5 mm inferior to mid-anterior/posterior commissure (ACPC);58 and 12.12 lateral, 2.41 posterior, and 2.39 inferior relative to the midcommissural point.59 Most of the reported locations of the STN target are posterior (1–3 mm) to the midcommissural point, though some authors report targets 4 mm anterior, 4 mm deep, and 12 mm lateral to the midcommissural point.60 Using a statistical correlation of the coordinate values of active electrode contacts with the amplitude of residual clinical symptoms and side effects in a cohort of 41 patients treated by STN DBS for PD, Geuhl et al61 showed that the optimal target is located 12–12.3 mm lateral to the ACPC line and 3.1 to 3.3 mm under the ACPC line; no preferred y-coordinate location (distance in front or behind the midcommissural point) could be found with this method.
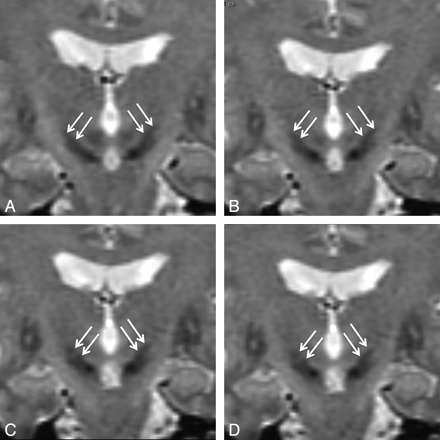
Many authors use direct MR imaging targeting of the STN. The use of coronal T2-weighted images to visualize the STN was first described by Bejjani et al.62 These authors showed that the STNs were visible as biconvex hypointense structures located in the upper mesencephalon (Fig 2). They also proposed using the anterior border of the red nucleus as an internal reference for the anteroposterior location of the STN target. An anatomic MR imaging study63 has defined the spatial distribution of the STN, showing that the hypointense signal intensity located lateral to the red nucleus and dorsolateral to the substantia nigra was correlated with the presence of iron and corresponded anatomically to the STN. This study also showed that at 1.5T, the MR imaging T2 hypointensity predominated in the rostral two-thirds of the STN and that the posterior part of the nucleus was not hypointense and thus not visible in most cases.
Coronal T2-weighted images showing the STN in a patient with advanced PD (spin-echo acquisition; TR/TE/NEX, 2200 ms/90 ms/2). Section thickness is 2 mm, located every 1 mm. A, The section is located 1 mm behind the anterior limit of the red nuclei. Both STNs are seen as almond-shaped hypointense structures above the locus niger (arrows). B, This section is located 1 mm in front of A, at the level of the anterior limit of the red nuclei. Both STNs are clearly seen (arrows). C and D, These sections are located 1 and 2 mm in front of B, showing the anterior extension of the STN (arrows).
The use of direct visualization of the STN by MR imaging, coordinate guidelines, and intraoperative microelectrode recording allowed precise electrode implantation in 27 patients.64 It has been shown that 3T MR imaging allows direct visualization of the STN on contiguous high-resolution T2-weighted fast spin-echo images.58 Other authors compared the location of the STN as obtained by direct localization at 3T with a computer-aided atlas-based procedure for automatic STN identification and showed that there was good agreement between the direct identification of the STN at 3T and the automatically identified structures.65
More recently a multi-gradient-echo fast low-angle shot technique to visualize the STN,66 exploiting the increased sensitivity of T2* to local iron deposits, has been used. This 3D MR imaging technique enables simultaneous acquisition of T1-weighted images for stereotactic use and images with superimposed T2* contrast to localize the STN.
Some authors showed that direct-target planning on MR imaging was more reliable than targeting based on atlas-derived data.57 Others showed that the best targeting method, according to their data, was a direct MR imaging targeting technique by using the red nucleus as an internal fiducial marker. These authors showed that the use of 3D MR imaging reconstruction allowed better targeting than 2D MR imaging.67
GPi Targeting.
The use of 2-mm-thick contiguous axial turbo spin-echo proton attenuation−weighted images68 has been described to visualize the boundaries of GPi, globus pallidus externus, and lamina medullaris interna in 48 patients, allowing a clear determination of anatomic boundaries in 71% of the patients. The target point was chosen at the center of the visualized posteroventral pallidum, irrespective of the position of this point in relation to commissures. GPi targeting has been described using direct 3D T1-weighted MR imaging in children with dystonia.69,70 Significant differences have been reported between atlas- and MR imaging−determined targets for this treatment indication. MR imaging targeting was validated by postoperative clinical findings indicating that MR imaging targeting is more precise than atlas-based targeting for GPi in children.70
VIM Targeting.
The VIM nucleus of the thalamus, like most thalamic subdivisions, is not directly visible on MR imaging. Targeting of the VIM nucleus on stereotactic MR imaging has been described by using an analytic determination of the Guiot parallelogram. This geometric construct represents the spatial extension of the VIM nucleus in a parasagittal plane. It was first described for stereotactic thalamotomy and was originally performed as a schematic drawing on ventriculography.71 Its limits are defined by using the ACPC length, the stereotactic coordinates of AC, PC, the midline sagittal plane, and the thalamic height. The stereotactic coordinates of these structures can be easily obtained on 3D stereotactic MR imaging,72 allowing calculation of the geometric extent of the parallelogram, so the location of the nucleus can be inferred.
Intraoperative Confirmation of Electrode Placement
Radiologic Confirmation
Radiologic control during the electrode implantation procedure for DBS is used by most surgical teams. Radiographs are obtained during the surgical procedure to confirm that the exploratory or the definitive electrodes are precisely following the predetermined trajectory.72 More recently, the use of intraoperative CT73 or MR imaging52 to check electrode positioning has been described.
Electrophysiology
The use of intraoperative electrophysiologic examination during DBS electrode placement remains controversial. For example, during STN electrode implantation, complete electrophysiologic mapping62 of the anatomic target by multiple microelectrodes to confirm the position of the therapeutic target is considered by some neurosurgeons as mandatory, whereas others prefer to limit the electrophysiologic study to reduce the duration of the intervention and limit or eliminate the risks of this type of study.74,75
Postoperative Imaging
Postoperative evaluation of patients with implanted electrodes is used to confirm the absence of complications. CT can detect most implantation-related complications. However, postoperative MR imaging may be more sensitive to some complications, like electrode-related infections. MR imaging also more precisely localizes the position of the contacts of the implanted electrodes. However, there is a risk of electrode heating when MR imaging is performed in patients with DBS systems because of electric current induced by radio-frequency electromagnetic waves.76,77
The Risks of MR Imaging in Patients with DBS Systems
Two cases of MR imaging−related accidents in patients with neurostimulation systems, 1 reversible and the other leading to irreversible brain damage and significant clinical sequelae have been published.78,79
The principal manufacturer of DBS systems states that MR imaging is contraindicated in patients with DBS unless specific precautions are taken. If these precautions are taken, MR imaging can and has been performed in many cases safely, but the patient and physician must be aware of the potential MR imaging hazards. The most important precautions (this is not an exhaustive list) that must be taken when performing MR imaging in these patients are the following: 1) Use a 1.5T MR imaging system; 2) stop the DBS stimulation for the duration of the scanning; 3) use only a transmit-receive-type radio-frequency head coil (not a whole-body radio-frequency coil, a receive-only head coil, or a head-transmit coil that extends over the chest area); and 4) select MR imaging parameters with a specific absorption rate (SAR) that does not exceed 0.1 W/kg in the head.
These conditions are extremely restrictive. For example, in these patients, it is only possible to perform MR imaging with a head coil. This means that examination of any other part of the body with MR imaging is contraindicated. Moreover, in a patient with a DBS system, MR imaging of the brain is contraindicated if a transmit-receive head coil is not available. With the recent evolution of MR imaging systems and the tendency to use receive-only multichannel head coils, the use of MR imaging in these patients will become more and more problematic. Neuroradiologists must be aware of these potential hazards, and a history of prior DBS implantation must be systematically sought before MR imaging studies, as part of routine screening for implanted devices and foreign objects. The SAR data obtained with MR imaging systems are only estimates, so the manufacturer's suggestions are only guidelines. A recent study using temperature measurement inside a head phantom80 has shown that the ratio of the actual average head SAR to the scanner-displayed value may vary between 0.3 and 2.1. However, according to these authors, in practice, because not all combinations of transmit gain and patient weight are encountered, a narrower range of coil correction factors (eg, from 0.5 to 1.0) would be encountered.
MR imaging electrode heating has been studied in vitro at 1.5T by using a gel-filled phantom.81 The conclusion of this study was that temperature elevations associated with clinical sequences were within an acceptable physiologically safe range. However, it must be stressed that these findings are specific to the neurostimulation systems, device-positioning technique, MR imaging system, and imaging conditions used in the study.
More recently, some authors investigated safety issues when performing functional MR imaging (fMRI) investigations in human subjects with fully implanted active DBS systems.82 The study was performed at 1.5T and 3T by using head-transmit coils. The authors showed that for fMRI sequences with coil-averaged SARs <0.4 W/kg, MR imaging−induced temperatures were less than measurement sensitivity (0.1°C) at 1.5T and <0.5°C at 3T. MR imaging pulse sequences with SARs of 1.45 W/kg at 1.5T and 2.34 W/kg at 3T led to temperature increases >1°C (ie, greater than those considered safe for human subjects).
Despite these potential hazards, some authors have reported the use of MR imaging to control the depth of electrode implantation. Postoperative MR imaging is the most precise imaging method to localize electrode contacts.
Postoperative Evaluation of Complications
Reported intra- and immediate postoperative complications are hemorrhage and ischemia.83 Binder et al84 systematically studied the hemorrhagic risk of DBS electrode implantation by using CT or MR imaging in a series of 481 electrode implantations in the STN, ventrolateral thalamus, and GPi. Hematomas were observed in 16 patients, and these were symptomatic in 6 patients. Only 3 patients among the 6 symptomatic cases or <1% of the total had permanent new neurologic deficits. Transient confusional states have been reported in 5%–25% of patients, following bilateral subthalamic electrode implantation for PD.85 Lyons et al,86 in a prospective series of 81 patients treated with bilateral STN electrode implantation, reported no serious surgical complications resulting in death or permanent neurologic deficit, with just 1 intracranial hemorrhage. During follow-up averaging 17 months, there was a 2.5% rate of infection requiring system removal, 3.7% rate of infection requiring implantable pulse-generator removal, a 12.5% rate of misplaced leads, and a 26.2% rate of hardware complications (lead migration, lead fracture, and malfunction of the implantable pulse generator).
Long-term complications of DBS are mainly hardware-related complications (ie, infection, malfunction, and lead migration or fracture). These complications have been prospectively studied in a series of 144 patients from 2 different Canadian centers.87 Complications related to the DBS hardware were seen in 11 patients (7.6%). There were 2 lead fractures (1.4%) and 9 infections (6.2%).
Cognitive Side Effects of DBS
There is a growing number of reports that DBS may result in psychiatric complications. These adverse events have mainly been reported for DBS in PD with the STN target.88 Depression, hypomania, euphoria, and hypersexuality have been described following DBS procedures.88 A higher-than-expected frequency of suicide has also been reported among patients undergoing STN DBS for advanced PD.89 In a prospective study of 20 patients during the 2 years after surgery, however, Houeto et al90 showed that provided patients with PD are rigorously selected for neurosurgery, STN stimulation improves mood, anxiety, and quality of life. The procedure does not result in severe permanent psychiatric disorders or modify patients' personalities, and it does not improve social function.
Impulsivity has also been described as a cognitive side effect in DBS. In a very interesting article, Frank et al91 showed that DBS of the STN selectively interferes with the normal ability to slow down when faced with decision conflict. Under high-conflict conditions, patients on DBS sped up their decisions.
The results of a randomized study92 comparing DBS with the best medical treatment for PD according to the German Society of Neurology guidelines show that there is a selective decrease in frontal cognitive functions and an improvement in anxiety in patients after the treatment. However, these changes did not affect improvements in quality of life, and there was no overall reduction of cognition or mood.
Study of Electrode Contact Positions on Postoperative Imaging
Imaging of electrode contact position after DBS electrode implantation provides important data confirming the relationship between the electrode and the target, including the precision of targeting. Imaging can also be used to check the exact position of the contacts in cases of clinical failure of DBS. Usually, contact location is determined by means of atlas registration on postoperative MR imaging or CT. MR imaging is the most precise tool for contact-position evaluation but is not always feasible for safety reasons.
Using the Schaltenbrand and Wharen atlas fused on postoperative MR imaging in patients treated with GPi DBS for PD, Yelnik et al22 demonstrated a contrasting effect on akinesia and rigidity of stimulation in the internal and external pallidum in PD. Study of the localization of STN electrodes in patients with PD with a 3D atlas−MR imaging coregistration method93 has shown that though the STN was the most efficacious target for DBS treatment in PD, stimulation of surrounding regions (zona incerta or the lenticular fasciculus) could also improve symptoms of PD. With the same method, in patients treated for dystonia by using GPi DBS, it has been possible to study the functional map of the globus pallidus (GP), showing that bilateral acute ventral stimulation of the GP significantly improved the dystonia, whereas bilateral acute dorsal pallidal stimulation, primarily localized within the external GP, had variable effects across patients. Half of patients demonstrated slight or no improvement or even aggravation of dystonia compared with baseline.94
Some authors56 studied the distribution of the most clinically effective contacts of STN electrodes defining a “probabilistic functional atlas.” This probabilistic atlas is based on the results of 168 bilateral subthalamic stimulations mathematically combined to define an idealized common space. They found a functional volume of 240 mm3 for the left and 229 mm3 for the right STN. Defining the region of the highest probability as the “hot STN,” they found a value of 5.52 mm3 for the left and 3.92 mm3 for the right hot STN. More recently, these authors compared the anatomic and functional human STN by using the same atlas and showed that the functional STN and the anatomic STN correlated well for medium and high probabilities of the functional STN.95
A histologically based deformable 3D atlas of the basal ganglia was used on a series of patients with parkinsonism treated by DBS,55,96 allowing comparison of atlas data with postoperative stimulation results (Fig 3).
Postoperative study of the position of electrode contacts in a patient with bilateral implantation of electrodes in the STN for the treatment of PD. Postoperative 3D MR imaging acquisition with fusion of anatomic data by using a histologically based deformable 3D atlas of the basal ganglia (Yelnik et al55). A, 3D posterior oblique view shows the caudate nuclei (blue), the STN (pink), the locus niger (black), and the electrode contacts (yellow, active contacts; blue, inactive contacts; contacts inside or behind the STN are seen in the transparency). B, Frontal view through the active contact. The exact position of the contacts inside the metallic artifact on the MR imaging acquisition is indicated on each side by a yellow dot. Both contacts are located inside the STN (pink). Anatomic limits of the putamen (blue), caudate (blue), locus niger (black), thalamus (green), and optical tracts (orange) on the basis of the 3D atlas are shown. C, Axial view through the right active contact (yellow dot; the left active contact is above and is not seen in this view). The right active contact inside the STN (pink), caudate, putamen, and red nuclei (orange) can be seen. D, Right sagittal view through the right active contact (yellow dot). The same color code is used for caudate, thalamus, locus niger, and optical tract (orange).
The use of postoperative imaging is helpful when a failure of the DBS procedure is observed. Okun et al97 studied 41 consecutive patients with suboptimal results from DBS surgery performed in other centers. These authors showed that good outcomes could be obtained in 51% of these patients after appropriate intervention. The main causes of the failures were suboptimally placed electrodes (46%), suboptimal pacemaker programming (54%), and suboptimal medical treatment (73%).
Another study of STN implantation failure has recently demonstrated that precise location of the stimulating electrode is essential and that misplacement of electrodes is a possible explanation for suboptimal response to bilateral STN stimulation in patients with PD.98 Seven patients who experienced persistent motor disability despite bilateral STN stimulation underwent reimplantation, and all patients except 1 showed improvement after the repeat procedure. STN stimulation improved the basal state Unified Parkinson's Disease Rating Scale motor score by 26.7% before reimplantation and by 59.4% at 1 year after reimplantation. The median levodopa equivalent daily dose was reduced from 1202 mg to 534 mg. The mean distance between the contacts used for chronic stimulation and the theoretic effective target as defined by these authors decreased from 5.4 to 2.0 mm, suggesting that there is probably an optimal anatomic target for STN stimulation in PD and that a relatively small spatial adjustment of the electrode can lead to a significant clinical improvement.
DBS and Imaging: Understanding DBS Mechanisms and the Human Brain
Careful postoperative clinical observation of patients with DBS has lead to very important and unexpected discoveries in the functioning of the human brain. In some cases, these have resulted in the development of new treatment strategies for incurable neurologic or psychiatric diseases. These effects can be observed during the intervention, when stimulation is performed to check the position of the electrode or they can be noted postoperatively. The effect can be acute or chronic; it can be due to stimulation of the targeted nucleus or stimulation of other nuclei located near the target. The postoperative stimulation of nuclei near the target is possible because stimulating electrodes used in DBS have 4 contacts, permitting a choice of the most effective sites of stimulation in the target region. During the postoperative period, the effect of chronic stimulation of each contact is evaluated to detect the best therapeutic contact. Unexpected effects observed during stimulation of contacts located outside the therapeutic target can demonstrate correlation of neuronal network activation or inhibition with patient behavior. When such an effect is observed, a high-resolution MR imaging (or CT) anatomic correlation can be obtained to determine the exact location of the electrode contact responsible for the unexpected effect. Many interesting observations have been published about these effects. We will highlight just a few of the more striking results.
Intraoperatively, in a patient treated with bilateral hypothalamic DBS for morbid obesity, it was observed that stimulation evoked detailed autobiographic memories.99 This was attributed using electroencephalographic source localization to be related to activation of mesial temporal lobe structures. Others reported correlation of obsessions, with hyperactivity of the caudate nucleus observed during intraoperative electrophysiologic study in patients treated for severe forms of OCD by using DBS of the caudate nucleus.100
During the postoperative period, it has been shown that DBS induced reversible acute depression101 in the case of a 65-year-old woman treated with STN DBS for PD, who had no history of psychiatric disorders. In this patient, stimulation of the lowest contact of the left electrode (the electrode contact located 3 mm above was therapeutic) induced acute reversible depression. The patient started to cry and verbally communicated feelings of sadness, guilt, uselessness, and hopelessness. The syndrome elicited by stimulation fulfilled all the criteria of the Diagnostic and Statistical Manual of Mental Disorders except (because stimulation of this contact was only maintained for a few minutes) weight change and sleep disorder. The syndrome resolved within a minute after stimulation ceased. High-resolution study of the position of the responsible electrode contact showed that it was located in the central part of the substantia nigra. The therapeutic contact was in the STN and did not modify the patient's mood. Positron-emission tomography (PET) performed during electrode stimulation showed activation of the left orbitofrontal cortex, a finding consistent with involvement of the nigrothalamic pathway.
Pathologic crying was observed in a 48-year-old woman with advanced PD who received bilateral implantation of deep brain stimulators in her STN.102 Stimulation resulted in pathologic crying. This effect was observed during her postoperative evaluation. Estimation of the position of the involved contact showed that it was located in the region of the caudal internal capsule. The patient did not know why she was crying and could not stop herself from crying. There was no sensation of sadness, pain, or persecution. The most interesting observations were those of patients having both PD and OCD who were treated by bilateral STN stimulation.19,20
In 3 cases, DBS stimulation produced dramatic amelioration of both motor and OCD symptoms, with disappearance of compulsion and significant improvement of obsessive symptoms. This is concordant with pathophysiologic models, suggesting that OCD might be associated with dysfunctions in corticostriatopallidothalamocortical neuronal circuits. These observations suggesting that STN DBS could be used in severe medically intractable OCD were confirmed by a multicenter study assessing the efficacy of the stimulation of the STN in OCD.21
In a highly precise clinical anatomic correlation study by using an interactive brain atlas, Mallet et al103 studied 2 patients with parkinsonism who experienced transient hypomanic states after STN electrode implantation for DBS. Functional neuroimaging studies of these patients with PET during stimulation of the contacts that produced the hypomanic state demonstrated that stimulation was concomitant with activation of cortical and thalamic regions known to process limbic and associative information. Study of the localization of the electrode contacts showed that the hypomanic state was caused only by stimulation through 1 contact localized in the anteromedial STN and that both this contact and the contact immediately dorsal to it improved the parkinsonian motor state. These authors proposed a model in which the 3 functional domains, emotional, cognitive, and motor, can be subtly combined in the small volume of the STN. They suggested that this nucleus could be a nexus that integrates the motor, cognitive, and emotional components of behavior.
fMRI in Patients with DBS
fMRI has been described during DBS in patients with electrodes implanted in the STN. This study was performed after extensive phantom safety testing of DBS lead systems. An fMRI study at 3T in 5 patients showed activation in the ipsilateral basal ganglia in all subjects and in the ipsilateral thalamus in 6 of the electrodes tested. Two of the stimulation electrodes demonstrated additional activation in the STN and/or substantia nigra region adjacent to the electrode tip.104
Other authors, however,105 reported potentially significant heating, high induced voltage, and even sparking at defects in the connecting cable in a phantom study performed to evaluate the feasibility of active DBS during fMRI. These authors concluded that there were severe potential hazards for patients but that under certain conditions, safe MR imaging examinations during active DBS was feasible. Another phantom study demonstrated that false-positive activation could be observed on fMRI during DBS.106
PET Studies in Patients with DBS
Because of the reported potential hazards related to fMRI, PET is the preferred functional method for studying patients during DBS. PET can be used to understand the mechanism of DBS or to study unexpected effects of DBS.
With PET, it has been shown107 that STN stimulation induced a significant diminution of PD-related covariance patterns of regional metabolism. There was a DBS-induced diminution of metabolism in the GPi and caudal midbrain. However, this correction of abnormal network activity in PD with DBS has been observed for motor systems, but not for cognitive networks.108
PET studies have also demonstrated that DBS of the STN leads to task-specific modifications of neural activity, with appropriate recruitment of motor areas and widespread nonspecific reductions of compensatory or competing cortical activity.109 Other authors showed that that there was a strong positive correlation of relative cerebral blood flow to increasing stimulation frequency around the STN and that the gradual increases in STN stimulation frequency were tightly correlated with decreases in motor cortex activity.110
Conclusions
DBS is an actively developing field. There is a rapidly increasing number of patients who are treated with DBS, mainly for PD. Many new targets and new applications are emerging for neurologic and psychiatric indications like intractable OCD and depression. Neuroimaging is extremely important for the management of these patients in the preoperative, perioperative, and postoperative timeframe. Future research is needed to improve targeting techniques and to develop safe methods to determine the precise anatomic location of electrode contacts.
Indicates open access to non-subscribers at www.ajnr.org
References
- 1.↵
- 2.↵
- 3.↵
- 4.↵
- 5.↵
- 6.↵
- 7.↵
- 8.↵
- 9.↵
- 10.↵
- 11.↵
- 12.↵
- 13.↵
- 14.↵
- 15.↵
- 16.↵
- 17.↵
- 18.↵
- 19.↵
- 20.↵
- 21.↵
- 22.↵
- 23.↵
- 24.↵
- 25.
- 26.
- 27.
- 28.
- 29.↵
- 30.↵
- 31.↵
- 32.↵
- 33.↵
- 34.↵
- 35.↵
- 36.↵
- 37.↵
- 38.↵
- 39.↵
- 40.↵
- 41.↵
- 42.↵
- 43.↵
- 44.↵
- 45.↵
- 46.↵
- 47.↵
- 48.↵
- 49.↵
- 50.↵
- 51.↵
- 52.↵
- 53.↵
- 54.↵
- 55.↵
- 56.↵
- 57.↵
- 58.↵
- 59.↵
- 60.↵
- 61.↵
- 62.↵
- 63.
- 64.↵
- 65.↵
- 66.↵
- 67.↵
- 68.↵
- 69.↵
- 70.↵
- 71.↵
- 72.↵
- 73.↵
- 74.↵
- 75.↵
- 76.↵
- 77.↵
- 78.↵
- 79.↵
- 80.↵
- 81.↵
- 82.↵
- 83.↵
- 84.↵
- 85.↵
- 86.↵
- 87.↵
- 88.↵
- 89.↵
- 90.↵
- 91.↵
- 92.↵
- 93.↵
- 94.↵
- 95.↵
- 96.↵
- 97.↵
- 98.↵
- 99.↵
- 100.↵
- 101.↵
- 102.↵
- 103.↵
- 104.↵
- 105.↵
- 106.↵
- 107.↵
- 108.↵
- 109.↵
- 110.↵
- Copyright © American Society of Neuroradiology