Abstract
BACKGROUND AND PURPOSE: During the hyperacute phase of stroke, multiple hypointense vessels were identified specifically in the ischemic territory on gradient-echo T2*-weighted MR images (GRE-T2* WI) at 3T. The area was named a “region of multiple hypointense vessels (RMHV).” The aim of this study was to assess the usefulness of RMHV for the diagnosis of acute ischemic stroke (AIS) and to establish the relationship of this finding to other MR imaging studies.
MATERIALS AND METHODS: Twenty patients with AIS underwent MR imaging at 3T consisting of GRE-T2*, diffusion-weighted images (DWI), and perfusion-weighted images (PWI) within 6 hours of symptom onset and follow-up images at 72 hours. RMHV was defined as an area containing multiple hypointense vessels strictly in the region of the ischemic territory on GRE-T2*. The RMHV volume on GRE-T2*, initial ischemic lesion volumes on DWI, PWI maps, and on follow-up images were measured and compared with the RMHV volume.
RESULTS: RMHV on GRE-T2* was identified in 20 patients. There was no significant difference between the ischemic lesion volumes on mean transit time (247.3 ± 88.1 mL), time-to-peak (228.6 ± 88.8 mL), cerebral blood flow (200.6 ± 89.7 mL), RMHV on GRE-T2* (214.4 ± 86 mL), and the infarct volume at 72 hours (210.3 ± 90.4 mL) (P = .975).
CONCLUSIONS: RMHV on GRE-T2* can be used as a supportive imaging finding for the diagnosis of hyperacute ischemic stroke. RMHV volume provides information that is in accordance with the infarct volume at 72 hours and the data supplied by PWI.
Recent studies have demonstrated that multiparametric MR imaging protocols offer an additional source of data regarding tissue status and identify patients with stroke who may benefit from intravenous thrombolysis. Gradient-echo T2*-weighted image (GRE-T2* WI) is a sequence sensitive to paramagnetic substances such as deoxyhemoglobin, which becomes a naturally occurring endogenous contrast agent for MR imaging.1,2 This MR image can be applied to exclude hemorrhage3 but can also show intra-arterial clot, which is seen as hypointense signal intensity in an artery and is called the GRE susceptibility vessel sign (GRE-SVS)4 in acute ischemic stroke (AIS). Furthermore “hypointense venous signal intensity loss area” has been described by use of 1.5 T GRE MR imaging in some cases of AIS in animals5 and human studies6-8 related to the abnormal visibility of transcerebral veins or abnormal visualization of leptomeningeal vessels. We observed a similar finding of multiple venous or arterial hypointense vessels strictly confined to the ischemic territory in all patients who had large cerebral arterial occlusion in the hyperacute phase of the ischemic stroke at 3T GRE MR imaging.
The area containing the linear and branching signal intensity–void structures on GRE-T2* was named the region of multiple hypointense vessels (RMHV). The main aim of this study was to assess the usefulness of RMHV for the diagnosis of acute ischemic stroke and to determine the relationship of this finding to other established MR studies in AIS.
Materials and Methods
Patients
From November 2006 to August 2008, a total of 67 consecutive patients with acute stroke were evaluated with 3T MR imaging protocol within 6 hours after symptom onset. The following data were recorded: patient age, sex, location of infarction, and time of onset of clinical symptoms at admission to the hospital. Stroke severity was assessed according to the National Institutes of Health Stroke Scale (NIHSS) score. The following criteria were established for inclusion in the study: 1) baseline NIHSS score of more than 4, and 2) large-artery occlusion on time-of-flight MR angiography (TOF-MRA). Intravenous (IV) thrombolysis with recombinant tissue plasminogen activator (rtPA) was given within 3 hours after symptom onset at the usual dose of 0.9 mg/kg.9 Eligibility criteria for rtPA administration at our hospital included the conventional National Institute of Neurologic Disorders and Stroke criteria9 (single exception: patients older than 80 years were not treated by rtPA), with the addition of MR imaging criteria (perfusion/diffusion mismatch). Informed consent was obtained from patients or their relatives. This prospective study protocol was approved by the hospital ethics committee. There were 30 of 67 patients who had acute large cerebral artery occlusion, 7 of 30 patients had transient ischemic attack, and 3 of 30 patients had movement artifact on the MR imaging sequences. Therefore, only 20 patients were finally included in the study. There were 12 of 20 patients who were admitted to the hospital within 3 hours after symptom onset and 8 of 20 patients within 3 to 6 hours. Six of 12 patients were treated with IV rtPA, but the other 6 patients were not treated with rtPA because perfusion/diffusion match was observed in 2 patients, a co-incidental aneurysm was found in 1 patient, a large diffusion-weighted image (DWI) lesion was observed in 1 patient, malignant hypertension (240/130 mm/Hg) was found in 1 patient, and the sixth patient was 87 years old.
Imaging Sequences
3T MR imaging (Trio; Siemens, Erlangen, Germany) acute stroke protocol parameters were as follows: 3D TOF-MRA: TR, 19 ms; TE, 3.69 ms; flip angle (FA), 18°; matrix, 177 × 256; FOV, 165 × 220 mm; partition/slab thickness, 64/72 mm; NEX, 1; acquisition time (AT), 71 s. The parameters for the turbo spin-echo (TSE) T2WI were TR, 3500 ms; TE, 85 ms; matrix, 153 × 256; FOV, 178 × 220 mm; NEX, 1; AT, 40 s. The parameters for the TSE T1WI were TR, 400 ms; TE, 12 ms; matrix, 144 × 256; FOV, 177 × 230 mm; NEX, 1; and AT, 36 s. The parameters for the fluid-attenuated inversion recovery (FLAIR) images were TR, 7620 ms; TE, 82 ms; matrix, 156 × 256; FOV, 177 × 230 mm; NEX, 1; and AT, 70 s. The imaging parameters for the GRE-T2* fast low-angle shot (FLASH) images were TR, 400 ms; TE, 12 ms; matrix, 180 × 320; FOV, 177 × 230 mm; NEX, 1; and AT, 74 s. The parameters for the contrast-enhanced cervical MR angiography (MRA) 3D FLASH T1 images were TR, 2.91 ms; TE, 1.2 ms; matrix, 256 × 300; FOV, 225 × 300 mm; FA, 15; voxel size, 0.8 × 0.8 × 0.8 mm3; bandwidth, 930 Hz/px; sections per slab, 80; partition thickness, 0.8. The imaging parameters for the DWI studies were TR, 3100 ms; TE, 106 ms; matrix, 128 × 128; FOV, 196 × 196 mm; NEX, 3; AT, 85 s; and 2 b-values (1000 and 2000 s/mm2). The latter applied in each of the 3 principal gradient directions (x, y, and z) were used to calculate the apparent diffusion coefficient (ADC). We obtained perfusion-weighted images (PWI) using a bolus of gadobenate dimeglumine (MultiHance; Bracco Diagnostics, Princeton, NJ), 0.1 mmol/kg injected at 4 mL/s, and followed by 30 mL of saline. PWI was performed with spin-echo echo-planar imaging (EPI) sequence with the following parameters: TR, 1360 ms; TE, 31 ms; matrix, 128 × 128; FOV, 230 × 230 mm; NEX, 1; and AT, 78 s. We obtained parametric perfusion maps including mean transit time (MTT), time-to-peak (TTP), and relative cerebral blood flow (rCBF) and volume (rCBV) using commercially available software supplied by the vendor. After a suitable basic image for positioning of the arterial input function (AIF) is selected, region of interest is drawn on healthy arteries close to the perfusion anomaly. The anterior cerebral artery (ACA) is usually selected for AIF. After selection of suitable AIF curves, the mean AIF was determined. The time range is set on the mean AIF curve. After the time range is confirmed, MTT, TTP, rCBF, and rCBV are calculated and displayed. All sequences were obtained with 5-mm section thickness and 1-mm intersection gap.
Baseline scanning protocol was standardized and included T2-WI, T1WI, DWI, TOF-MRA, FLAIR, and GRE-T2*. Baseline scans were obtained in all patients. If the patient had a large artery occlusion on TOF-MRA, PWI was added for evaluation of thrombolysis within 3 hours. To identify infarct volume at 72 hours, follow-up imaging with a T2-weighted MR imaging or 16-section row CT scanner (Sensation 16; Siemens, Malvern, Pa) was performed within 72 hours after symptom onset. All subjects had baseline and follow-up scans, but only 13 of 20 patients had initial PWI. Only 1 patient had an initial contrast-enhanced cervical MRA.
Image Analysis
Two independent experienced radiologists, A.D. (16 years’ experience) and M.E.Y. (7 years’ experience), blinded to each other's outlines and to clinical information concerning the patients visually identified and sequentially outlined regions abnormal on DWI (b = 2000), PWI, and GRE-T2* in baseline MR imaging and follow-up images (T2-weighted MR imaging in 14 patients and CT in 6 patients). Areas of increased signal intensity on DWI and decreased signal intensity on ADC maps were classified as acute infarct territory. Regions with hypoperfusion on PWI were identified as areas of increased signal intensity on MTT and TTP maps and as decreased signal intensity on rCBF and rCBV maps. RMHV was defined on the baseline GRE-T2*–weighted sequence as an area that contains multiple hypointense linear and branching vessels, extending through the cortex and subcortical regions of the ischemic territory (Figs 1 and 2). To identify the borders of the RMHV on GRE-T2* and measure the area containing these vascular structures, we marked the tips of the prominent vessels lying at the most medial, anterior, and posterior border, and we drew lines between these markings. Thus, segmentation was performed on each section where the prominent vessels were identified, and from the segmented areas, the total volume of the RMHV was calculated. Regions with increased signal intensity on follow-up T2-weighted images or decreased attenuation areas on CT scans were considered as the infarct region at 72 hours. All areas of ischemic territory were segmented on a section-by-section basis by manual editing. The volumes were automatically produced by the software (MRIcro; Chris Rorden, University of South Carolina, Columbia, SC) on the basis of the section thickness and overall outlined lesion areas. RMHV was absent on the normal contralateral hemisphere of patients with stroke. GRE-T2* images were also analyzed for the presence of SVS.
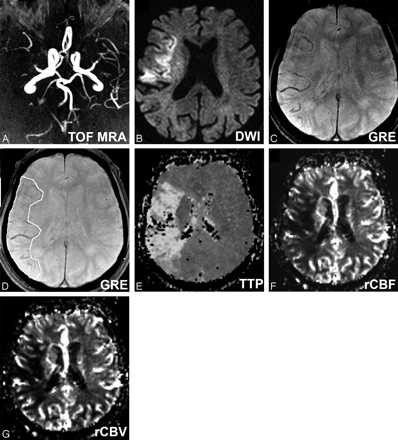
A, Craniocaudal collapsed MIP image of 3D TOF-MRA shows right MCA occlusion in case 2. B, Axial DWI-b2000 trace image shows hyperintensity in corresponding ischemic territory, C, Axial GRE-T2* shows RMHV as linear or branching structures strictly in the ischemic territory. Please note that the contralateral hemisphere is free of such structures. D, Axial GRE-T2* shows outlined RMHV on the same image. TTP (E), rCBF (F), and rCBV (G) maps show hypoperfusion area in corresponding ischemic territory.
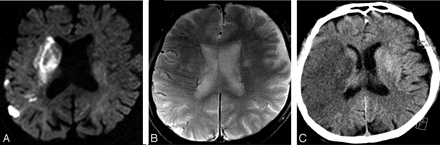
Right ICA occlusion in case 15. A, DWI-b2000 image shows small hyperintensities in the right posterior temporal and anterior parietal lobes, the caudate body and head, the right putamen and globus pallidus, and the internal and external capsules. B, Axial GRE-T2* corresponding image shows large hemispheric RMHV in the ischemic territory. C, Follow-up CT image shows the infarcted area at 72 hours.
Statistical Analysis
We analyzed the data using Statistical Package for Social Sciences (SPSS, Chicago, Ill) software (version 11.5). Friedman ANOVA was used for descriptive values and volumetric comparisons. Intraclass correlation coefficients (ICC) were computed to evaluate the correlation between examiners in lesion volume measurements on DWI, GRE-T2*, PWI maps, and follow-up images. The Bland-Altman plot was used for absolute difference between examiners.10,11 The Spearman correlation coefficient was used to analyze the relationship between lesion volumes and NIHSS score. The infarct volume at 72 hours of patients who received thrombolysis was not included for statistical analysis. Patient 5 had a large hemispheric hypoperfusion on PWI maps. The data of this patient were also not included in the statistical analysis because of acute occlusion of the angular branch (M3) of the middle cerebral artery (MCA) with concomitant ipsilateral severe stenosis of the proximal internal carotid artery (ICA). Patients were divided into 3 groups for volumetric measurement comparisons. The groups were structured according to availability of initial PWI:
Group 1 (patients 7–13) with initial DWI, PWI, GRE-T2*, and follow-up images.
Group 2 (patients 7–20) with initial DWI, GRE-T2*, and follow-up images.
Group 3 (patients 1–4 and patients 6–13) with initial DWI, PWI, and GRE-T2* images.
Results
The study population included 13 men and 7 women. The mean age of the study population was 71.8 years (range, 37–90 years), median initial NIHSS score was 13.14 (range, 6 –27). Symptomatic vascular occlusions were categorized as occurring in the ICA in 6 patients, in the MCA in 11 patients (M1, 6; M2, 1; M3, 4), in the ACA in 1 patient, in the posterior cerebral artery in 1 patient, and in the basilar artery in 1 patient as diagnosed on 3D TOF-MRA. SVS and RMHV were identified on GRE-T2* in all patients (Table).
Patient data and volumetric measurements
Group 1 (n = 7)
The mean (±SD) baseline whole DWI lesion volume was 91.6 ± 29.2 mL (range, 25.8–93.2 mL), MTT was 247.3 ± 88.1 mL (range, 52.3–259.2 mL), TTP was 228.6 ± 88.8 mL (range, 46.2–274.5 mL), rCBF was 200.6 ± 89.7 mL (range, 35.3–276 mL), rCBV was 175.3 ± 71.9 mL (range, 28.6–222.3 mL), RMHV on GRE-T2* was 214.4 ± 86 mL (range, 46.8–to 269 mL), and the infarct volume at 72 hours was 210.3 ± 90.4 mL (range, 49.5–284.7 mL). There was no significant difference between MTT, TTP, rCBF, and RMHV on GRE-T2*, and the infarct volume at 72 hours (P = .975).
Group 2 (n = 14)
The mean (±SD) baseline DWI lesion volume was 86.2 ± 41.5 mL (range, 16.3–176.2 mL), RMHV on GRE-T2* was 217.7 ± 74 mL (range, 46.8–269 mL), and the infarct volume at 72 hours was 214.5 ± 78.3 mL (range, 49.5–284.7 mL). There was no significant difference between RMHV on GRE-T2* and the infarct volume at 72 hours (P = .331).
Group 3 (n = 12)
The mean (±SD) baseline DWI lesion volume was 85.2 ± 27.5 mL (range, 25.8–106 mL), MTT was 256.3 ± 101.1 mL (range, 52.3–334.8 mL), TTP was 263.3 ± 99.4 mL (range, 46.2–329.3 mL), CBF was 214.4 ± 89.3 mL (range, 35.3–307.9 mL), and CBV was 174.4 ± 73.7 mL (range, 28.6–256.1 mL), and RMHV on GRE-T2* was 251.6 ± 92.6 mL (range, 46.8–311.5 mL). There was no significant difference on MTT, TTP, rCBF, and RMHV volume on GRE-T2* (P = .139) (Fig 3).
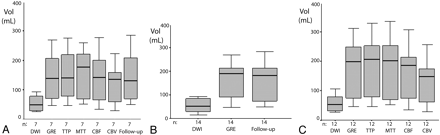
A, Baseline DWI, RMHV on GRE-T2*, TTP, MTT, rCBF, rCBV, and the infarct volume at 72 hours on follow-up images in group 1. There is no significant difference of volumes among TTP, MTT, rCBF, and RMHV on GRE-T2* and the infarct volume at 72 hours. B, Baseline DWI, RMHV on GRE-T2*, and the infarct volume at 72 hours on follow-up images in group 2. There is no significant difference of volumes between RMHV on GRE-T2* and the infarct volume at 72 hours. C, Volumetric measurement of lesions on baseline DWI, RMHV on GRE-T2*, TTP, MTT, rCBF, and rCBV in group 3. There is no significant difference of volumes among TTP, MTT, rCBF, and RMHV on GRE-T2*.
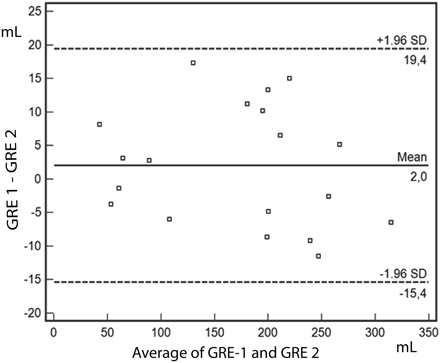
There was a high correlation between examiners performing volume measurements. The ICC values were 0.96 (P < .001) for DWI, 0.99 (P < .001) for MTT, 0.99 (P < .001) for TTP, 0.99 (P < .001) for rCBF, 0.98 (P < .001) for rCBV, 0.99 (P < .001) for RMHV on GRE-T2*, and 0.98 (P < .001) for the infarct volume at 72 hours. RMHV volume on GRE-T2* sequence correlation coefficient values (r) were 0.67 (P = .09) for DWI, 0.96 (P = .001) for MTT, 0.92 (P = .003) for TTP, 0.92 (P = .003) for rCBF, 0.96 (P = .001) for rCBV, and 0.91 (P = .001) for the infarct volume at 72 hours. NIHSS score correlation coefficient values were 0.55 (P = .19) for DWI, 0.66 (P = .10) for MTT, 0.61 (P = .14) for TTP, 0.61 (P = .14) for rCBF, 0.68 (P = .09) for rCBV, 0.73 (P = .058) for RMHV volume on GRE-T2*, and 0.73 (P = .058) for the infarct volume at 72 hours. The Bland-Altman plot shows the agreement between examiners performing ischemic volume measurement on TTP, rCBF, GRE-T2*, and the infarct volume at 72 hours. A total of 100% of data points were within the boundary limits for TTP, MTT, rCBF, RMHV on GRE-T2*, and the infarct volume at 72 hours on follow-up images and 83% for rCBV (Fig 4). The follow-up MR imaging included GRE-T2* only in 5 patients (patients 10–14) who did not receive thrombolysis. RMHV on GRE-T2* returned to normal within 48 hours after symptom onset in 4 of 5 patients.
Bland Altman plot of differences between 2 examiners. Dashed lines represent the mean (1.6 mL) and upper and lower boundaries. A total of 100% of data points are within the boundary limits for RMHV on GRE-T2*.
Discussion
The most important finding of our study was that RMHV was identified strictly in the ischemic territory in the hyperacute phase of stroke in all 20 patients on GRE-T2* at 3T. This sign on GRE-T2* can be used as a supportive finding in the radiologic diagnosis of acute stroke. The RMHV volume on GRE-T2* sequence correlated with MTT, TTP, rCBF, and rCBV lesion volumes on PWI and the infarct volume at 72 hours. There was no significant difference between the ischemic lesion volumes on MTT, TTP, rCBF, RMHV on GRE-T2*, and the infarct volume at 72 hours.
The SVS has been extensively studied in the literature. Cho et al12 have introduced the term SVS to describe the hypointense signal intensity within primarily occluded vessels on GRE-T2*. According to many studies, SVS on GRE-T2* represents a thrombus in an artery analogous to the CT hyperattenuated artery sign.13,14 We have also depicted SVS on GRE-T2* at 3T in all 20 patients with acute stroke. Hermier8 et al reported “abnormal visualization of leptomeningeal vessels” at 1.5T GRE in 17 of 48 patients with hemispheric ischemic stroke, which had no impact on clinical status and on the late infarct volume. We observed a similar finding of multiple hypointense vessels confined to the ischemic territory and that was not visible on the normal contralateral hemisphere, for which we preferred to use the term RMHV to emphasize this area containing multiple small venous or arterial vessels.
Increased oxygen extraction fraction in an ischemic brain with reduced cerebral blood flow15,16 leads to increased blood deoxyhemoglobin concentration in the cerebral veins and capillaries. Venous plus capillary fraction of cerebral blood volume is 63% to 70% of the total cerebral blood volume and contains higher concentrations of deoxyhemoglobin.17 Slow flow state may also contribute to an increased level of deoxyhemoglobin in the ischemic territory.18 The magnetic susceptibility difference between venous blood and the surrounding brain tissue makes it possible to visualize venous structures with use of GRE-T2* sequences.19 Reichenbach et al20 have demonstrated that multiple hypointense structures on MR venography at 3T were considered to be of mostly venous origin according to their anatomic distribution. However, multiple hypointense vascular structures may also include dilated capillaries or arterioles in the ischemic territory. Whether these vessels are dilated should be decided by angiography.
Prolongation of MTT secondary to slow flow has been observed as an early sign of ischemia such as in our series.21 RMHV volume on GRE-T2* was found to be larger than the DWI lesion volume within 6 hours and mostly disappeared within 48 hours (subacute phase) after symptom onset. There was no significant correlation between RMHV and NIHSS. Although there was no statistically significant difference in calculated lesion volumes on MTT, TTP, rCBF, RMHV on GRE-T2*, and the infarct volume at 72 hours, the results of our volumetric measurements showed minor variation. Baseline RMHV volume on GRE-T2* was smaller than baseline MTT and TTP lesion volumes. It has been already shown that MTT22 and TTP23 maps tend to overestimate the volume of tissue at risk for infarction.
PWI is a quantitative imaging technique for the prediction of final infarct volume.24 Our results showed that GRE-T2* can be used as an adjunctive or additive sequence to PWI. For example, patient 5 was admitted to our hospital within 80 minutes, presenting with a right-sided mild hemiparesis (NIHSS: 6). She had an acute occlusion of the left angular branch (M3) of the MCA with concomitant ipsilateral severe ICA stenosis. The DWI lesion volume of this patient was 6.1 mL, TTP was 281.4 mL, rCBF was 176.9 mL, and RMHV on GRE-T2* was 70.7 mL. We realized that acute occlusion of the M3 branch in this patient could not be responsible for the large hemispheric hypoperfusion. In conditions that are not straightforward, the GRE-T2* sequence may be helpful to determine the exact nature of an unperfused ischemic area.
Recent evidence suggests a potential link between the administration of gadolinium-containing contrast agents and nephrogenic systemic fibrosis/nephrogenic fibrosing dermopathy in patients with renal failure.25 GRE-T2* sequence can be a better choice in patients who have renal disease or a history of hypersensitivity to contrast agents. From a clinical point of view, RMHV on GRE-T2* depicts a region of hypoperfused tissue in acute stroke without the use of MR imaging contrast agents. Our data show that within a clinical routine setting of acute ischemic stroke evaluation, additional GRE-T2* sequence can supply an increased safety profile for the DWI/PWI mismatch concept. Combined DWI, PWI, and GRE-T2* sequences may produce valuable information in understanding the pathophysiology and treatment strategies of acute ischemic stroke. Haacke et al26 presented a new method of MR imaging, which they refer to as susceptibility-weighted imaging (SWI). The SWI technique is a high spatial resolution accelerated 3D T2*-weighted GRE sequence and has an advantage of depicting and enhancing magnetic susceptibility effects better than conventional T2*-weighted GRE sequence.27 This technique was unavailable during the period we performed our study.
Our study had some limitations. Although 1 month is the ideal time for final follow-up of infarct,28 we used 72 hours of follow-up imaging because most of our patients had acute large cerebral artery occlusions with severe disability. We preferred to perform imaging studies on those patients before they left the hospital. The infarct volume at 72 hours for patients who received thrombolysis was not included in the statistical analysis because the rtPA induced an improved effect on ischemic penumbra in the hyperacute phase. Seven patients outside of the thrombolysis window did not receive perfusion imaging. Furthermore, the number of our cases of stroke may be somewhat small, but of 67 cases, 20 patients who met the inclusion criteria and who were examined with advanced techniques within 6 hours were admitted to the study.
Conclusions
Our study indicates that the RMHV on GRE-T2* at 3T can be used as a supportive diagnostic imaging finding in acute ischemic stroke and that GRE-T2* is a useful sequence that provides information in accordance with PWI data.
References
- Received December 15, 2008.
- Accepted after revision January 8, 2009.
- Copyright © American Society of Neuroradiology