Abstract
BACKGROUND AND PURPOSE: With the approval of gadobenate dimeglumine, higher relaxivity MR contrast agents were introduced into the clinical environment, and multiple in vivo studies compared the efficacy and safety with the previously approved agents. An in vitro study was conducted to demonstrate differences between the various agents to confirm published values and for imaging-sequence optimization.
MATERIALS AND METHODS: A contrast phantom was made with serial dilutions of commercially available formulations of 5 US Food and Drug Administration–approved gadolinium-based MR imaging contrast agents in human serum substitute. Dilution factors ranging from 1:8 to 1:4096 were included in the phantom. Spin-echo sequences were performed at 1.5T and 3T with varying TRs and TEs.
RESULTS: At physiologic concentrations and by using short TRs and TEs, gadobenate demonstrated the highest signal intensities, confirming greater R1 relaxivity. At higher concentrations and with longer TR and TE values, the greatest signal intensity loss was appreciated for gadobenate, confirming greater R2 relaxivity.
CONCLUSION: Using rigorous in vitro methodology and serial dilution techniques, this study confirms the reported higher R1 and R2 relaxivities of gadobenate relative to the other agents at 1.5T and 3T.
With the development and subsequent introduction in June 1988 of gadopentetate dimeglumine, the first US Food and Drug Administration (FDA)–approved MR imaging contrast agent, clinical MR imaging underwent significant changes.1 Aside from the increased diagnostic yield of contrast-enhanced T1-weighted imaging (T1WI), it made way for the development of advanced imaging, including intravenous contrast bolus MR angiography and perfusion-weighted MR imaging (PWI) techniques. Several gadolinium-based MR imaging contrast agents were subsequently introduced into the clinical arena, but each was very similar to previously FDA-approved gadolinium-based MR imaging contrast agents (GBMCAs) in the mechanism of action, biodistribution, and biologic half-lives. They even shared similar safety profiles until the more recently discovered associations with the subsequent development of nephrogenic systemic fibrosis in patients with renal failure.2–5 Gadobenate dimeglumine, approved by the FDA in November 2004, represents the first FDA-approved GBMCA with noticeably higher R1 and R2 relaxivities and slightly different biodistribution and excretion pathways from those seen in the 4 older FDA-approved GBMCAs.6–8
Because of weak and transient protein binding, this agent has been reported to possess enhanced (R1 and R2) relaxivities and, therefore, faster transverse and longitudinal relaxation and recovery rates compared with the other FDA-approved GBMCAs.7–10 In clinical trials, it has been found to confer greater conspicuity and detectability to lesions within the central nervous system and associated cerebral vasculature, among other reported benefits.11–16 However, even in the intraindividual crossover studies, biodistribution of contrast within each patient, therefore the local concentration of contrast, cannot be determined or controlled. This was of particular interest, given the slightly different biodistribution reported for gadobenate relative to the other agents, with 3%–5% of the administered gadobenate dose undergoing biliary clearance.6,17–19 An in vitro study would potentiate more precise measurements of contrast concentration and correlation with corresponding signal intensities on any given MR imaging sequence. Such a study would allow more precise and quantifiable detection of differences between GBMCAs and optimization of imaging sequences to maximize the clinical benefits of each agent.
To this end, we designed an in vitro serial dilution study of each of the 5 FDA-approved GBMCAs currently available for clinical practice in the United States. These solutions would be simultaneously imaged with numerous MR imaging pulse sequences and imaging parameters to assess their relative signal intensities. These imaging studies would be repeated on both 1.5T and 3T MR imaging systems to evaluate the relative behavior of these agents at field strengths commonly used in clinical practice. It was hypothesized that these experiments would confirm published claims of relative relaxivities for these various agents and would demonstrate similar signal-intensity measurements at a given concentration, field strength, and pulse sequence for the older 4 GBMCAs and different values for gadobenate that would reflect its published higher R1 an R2 relaxivity values.
Materials and Methods
Serial dilutions of commercially obtained clinical use formulations of the 5 GBMCAs were performed by using Seronorm (Sero AS, Billingstad, Norway) human serum substitute as the diluent. Each of 10 serial dilutions was a 50% dilution of the prior concentration prepared into 5 mL of Seronorm within plastic 15-mL Falcon tubes (BD Biosciences, Franklin Lakes, NJ) to a maximal dilution factor of 1:4096. For each of the 5 GBMCAs studied, 10 tubes were imaged, representing dilution factors ranging from 1:8 to 1:4096, which were linearly arranged in their polystyrene holder in ascending order of dilution gradient. Several control test tubes of saline and other solutions were secured to the outside of the holder. Thus, the imaged phantom consisted of a polystyrene holder with 50 tubes containing 10 dilutions of each of the 5 FDA-approved GBMCAs, 1 agent per 10-tube column and progressively higher dilution factors in each row, with control solutions appended to the sides of the polystyrene holder.
Scanning Technique
The phantom was scanned by using axial image planes with FOVs of 28–32 cm, imaging matrix of 256 × 256, and section thicknesses of 5 mm. All imaging sequences were repeated in the identical fashion at both 1.5T and 3T at room temperature in clinical whole-body scanners (1.5T LX 9.1; 3T LX VH4; both GE Healthcare, Milwaukee, Wis). The phantom was leveled in the center of a transmit-receive head coil positioned at magnet isocenter. At each field strength, single-echo spin-echo MR imaging sequences were executed with varying TEs and TRs in a controlled fashion as outlined in Table 1. For each scanning session per field strength studied, center frequency and receiver and transmission gains were kept constant, being determined and fixed at long-TR test sequences.
Imaging parameters for spin-echo sequences performed at 1.5T and 3T
Measurements
Five axial images were acquired in multisection mode for each sequence. Of these, the middle image (image 3) was chosen for analysis so as to measure the region expected to have the most representative contrast concentration and minimal artifact from partial volume averaging for each tube studied. Images were imported into MIPAV software (http://mipav.cit.nih.gov/), and region-of-interest measurements were obtained from the center of each visible sample (Fig 1).20 Region-of-interest intensity and SD values were recorded for each of the 50 test tubes and controls on the selected central image of each of the sequences studied at both 1.5T and 3T. When the sequence produced no grossly detectable signal intensity within a given test tube, the position of the region of interest used to measure the signal intensity for that tube was selected by copying and pasting the position used in other sequences, which demonstrated signal intensity from that same tube. Data entry was double-checked for accuracy and reproducibility by repeating >10% of the measurements (randomly selected).
Sample image demonstrates the phantom at 3T, by using spin-echo technique (TR = 100, TE = 14).
Results
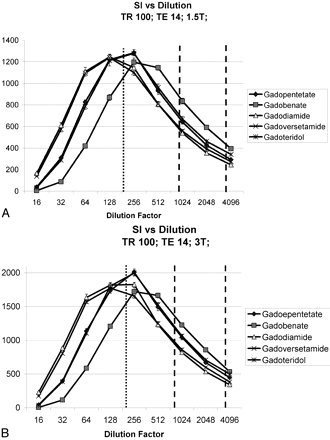
At TR values that were short relative to the anticipated T1 values of the materials tested, all contrast agents demonstrated the lowest intensities at the highest concentrations/lowest dilution factors studied. For all agents, signal intensities increased, peaked, and subsequently diminished as dilution factors ranged from high to low (Fig 2). The dilution factor at which signal intensity peaked varied across each GBMCA, TR, and TE studied. For example, at TR = 100 and TE = 14, peak signal intensity for gadodiamide was seen at a dilution factor of 1:128, whereas it peaked at 1:256 for gadobenate.
Measured signal intensity (SI) as a function of the dilution factor for TR = 100 and TE = 14 msec. At 1.5T (A) and at 3T (B), there are low signal intensities measured at low dilution factors/high concentrations, reflecting R2 relaxivity and T2(*) effects of contrast. At higher dilution factors/lower concentrations, T1 shortening effects predominate. Physiologic concentrations for T1WI range between the 2 dashed lines, 1:000–1:4000. Concentrations for PWI range below the dotted line, 1:200.
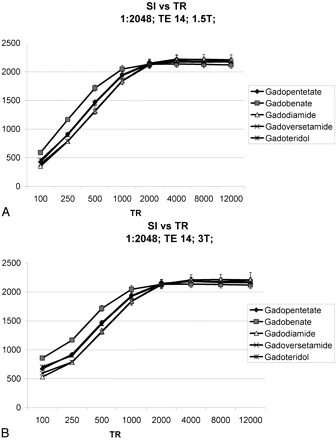
With parameters typical for clinical contrast-enhanced T1WI, Figure 2 shows that the highest signal intensities measured were for gadobenate, followed by gadopentetate and gadoteridol, followed by gadoversetamide and gadodiamide. Figure 3 demonstrates that at similar dilution factors, increasing TR yielded higher signal intensities for all 5 agents until it reached or exceeded 1000 ms. Although the relative order of signal intensities among the agents studies was maintained, the percentage increase in signal intensity (relative to the lowest measured value) decreased as the TR was lengthened.
Measured signal intensity (SIs) as a function of TR for TE = 14 ms and a dilution factor of 1:2048. At 1.5T (A) and at 3T (B), there is an increase in signal intensity with progressively longer TRs until full longitudinal magnetization recovery of the solution is reached, at approximately 2000 ms, for all agents.
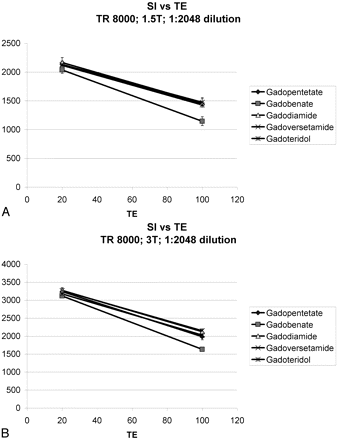
With low dilution factors typical for PWI, Figure 2 demonstrates that the lowest signal intensities and greatest T2(*) shortening effects were observed for gadobenate, followed by gadopentetate and gadoteridol, followed once again by gadoversetamide and gadodiamide. For PWI applications, plotting signal intensity versus TE for a given TR (Fig 4) demonstrated that the rate of signal-intensity decrease with increasing TE values was greatest for gadobenate. As T2-weighting was increased with longer TEs, signal-intensity loss was greatest for gadobenate. This was demonstrated by the use of 95% confidence intervals (Table 2), in which no significant differences existed between the measurements at TE = 20 and no overlap existed between the confidence intervals of gadobenate and the other agents at TE = 100. Most interesting, significant differences were also observed between gadoteridol and gadopentetate versus gadodiamide and gadoversetamide (Table 3).
Measured signal intensities (SIs) as a function of TE for TR = 8000 ms and a dilution factor of 1:2048. At 1.5T (A) and 3T (B), measured signal intensities are closely plotted at shorter TE values. With longer TE and greater T2-weighting, T2(*) effects of contrast predominate, and there is a decrease in measured signal intensity. The greatest signal intensity loss per TE is demonstrated for gadobenate.
T2(*) shortening effects as demonstrated by prolonging TE*
Effects of differences between perceived relaxivities among low relaxivity agents*†
By comparing signal intensities for a physiologic concentration with scanning parameters representative of contrast-enhanced T1WI at field strengths of 1.5T and 3T, Figure 5 demonstrates (as expected) higher signal intensities for all agents at 3T than at 1.5T. The relative benefit of gadobenate compared with the other agents was maintained at 3T as it had been seen at 1.5T. The signal-intensity benefit at 3T for gadobenate compared with the other agents was statistically similar to that seen at 1.5T for this agent compared with the other agents.
Measured signal intensities (SI) at 1.5 and 3T for TR = 250 ms, TE = 14 ms; 1:2048 dilution factor demonstrates globally increased SIs at a higher field strength.
Discussion
This study was performed to investigate the claim that gadobenate has uniquely higher R1 and R2 relaxivities when compared with the older FDA-approved commercially available GBMCAs. With an in vitro approach and serial dilution technique, a rigorous experimental methodology was expected to reveal any differences that may exist among these agents without subjecting the study to any of the variables that affect in vivo studies.
In humans, these agents all distribute throughout the extracellular fluid space, which corresponds to approximately 19–20 L of fluid in a 70-kg patient. As previously noted, 3%–5% of the administered dose of gadobenate undergoes biliary clearance.18 Discounting the effects of local concentration or renal clearance, standard doses of these agents to a 70-kg patient would correspond very roughly to a dilution of 14 mL into 20,000 mL, or ∼1:1,400-dilution value at equilibrium. Adding some of the effects of first-pass and clearance effects, approximate dilution values in most enhancing tissues in the first 5–10 minutes following administration, during which clinical imaging is typically performed, would correspond to contrast concentrations of very roughly between 1:1000 and 1:4000. In this physiologically functioning range, we find significant T1 shortening effects (which correspond with increased signal intensity on clinical T1WI) for each of the agents tested at 1.5T and 3T (Fig 2).
Although most clinical imaging takes place at “physiologic” dilution factors in the range of 1:000–1:4000, PWI, typically performed as a first-pass study, takes place at much lower dilution factors. During cerebral PWI, the entire brain is repeatedly examined every 2–3 seconds as contrast washes in, through, and then out of the tissues of interest. At such high concentrations, T2(*) effects of the infused contrast agents predominate. For sequences emphasizing T2-weighting, arrival of the first-pass contrast bolus, therefore, results in signal-intensity loss and returns to near baseline with washout of the agent from the vascular/capillary tree. Each of the agents demonstrates T2(*) shortening effects, illustrated by low measured signal intensities at low dilution factors (Fig 2).
Our data support the claim that when diluted in a protein-containing solution, such as the human serum substitute Seronorm, at identical concentrations, gadobenate yielded greater signal intensities at physiologic concentrations on typical T1WI sequences (ie, short TE, short TR sequences) than did the other 4 GBMCAs (Fig 3). Gadobenate also demonstrated even greater differences in its T2 shortening effects as demonstrated by lower signal intensities at given dilution factors as might represent those used in clinical PWI imaging and as represented by the greater observed negative slope in measured signal intensity with increasing TE values (Fig 4). For clinical application, this would allow smaller contrast doses yet equivalent contrast-to-noise ratios (CNR) compared with that produced at PWI with the other GBMCAs. Alternately, similar doses could provide greater CNR and increased sensitivity on PWI compared with other GBMCAs.
These data confirm the higher R1 and R2 relaxivities of gadobenate relative to the older agents, both at 1.5T as well as at 3T, in which the differences in measured signal intensities were even greater than those observed at 1.5T (Fig 5). With the increasing numbers of 3T scanners being installed, this observation has potential direct clinical significance as R1 relaxivity effects are converted into signal intensities and image contrast-to-noise ratios on diagnostic studies in humans. The higher signal intensity-to-noise ratios available at 3T compared with 1.5T are synergistically potentiated by the higher signal intensities measured at 3T compared with 1.5T in this study, which would translate into greater enhancement on clinical scans.
In a clinical setting, increased relaxivity can be converted into any of several clinical benefits. These include greater signal intensity-to-noise ratios and, therefore, CNRs between enhancing tissues and nonenhancing background structures, thus increasing resolving power and lesion detectability. These might be especially beneficial for detecting small structures/lesions, such as tiny metastatic foci and/or smaller vessels in contrast dynamic bolus MR angiographic techniques. Alternatively, lower administered doses might result in similar CNRs, thus providing a cost savings and/or increasing safety by permitting less of the agent to be administered to patients with, for example, renal disease. With a dose relationship now being established between the development of nephrogenic systemic fibrosis in patients with renal failure and total administered GBMCA, administering lower doses seems to provide a direct patient safety benefit in this regard.21 Additionally, scanning-parameter optimization for the higher relaxivity agent may yield its own benefits, such as shorter TR values, providing increasing CNRs in shorter scanning acquisition times. This is demonstrated in Figure 2, where the relative benefit of gadobenate over the other agents is maximized as TR is shortened, which also yields shorter scanning times and increased clinical scanner throughput. By switching from spin-echo to gradient-echo imaging, TR can be further shortened while maintaining T1 contrast by using minimal TE values and relatively larger flip angles.
A second, unexpected result relates to the older GBMCAs. It was initially accepted that the other 4 agents had essentially equivalent relaxivities and, as such, were largely interchangeable for clinical use. As demonstrated in Table 3, for a given concentration, gadopentetate and gadoteridol had statistically significant greater degrees of T1 shortening and, therefore, signal intensities than did gadodiamide and gadoversetamide at the same dilution factors. Although this unexpected finding is of unknown and questionable clinical significance, the observation remains of at least academic interest. Each of these agents had intensities and, therefore, relaxivities statistically significantly lower than those measured for gadobenate.
Limitations
All imaging was performed at room temperature, not at physiologic temperatures in a 37°C water bath. Despite our awareness of the fact that phantom temperatures play a role in determining the relaxivity and thus signal intensities observed, we recognized that this effect would be similar for each agent studied and therefore would not introduce any drug-specific bias into our results. As such, this would not be expected to impact the relative analyses performed in this study and would, therefore, not play a significant role in our attempt to assess relative behaviors of each of these agents in humans.
Conclusions
Using rigorous in vitro methodology and serial dilution techniques, this study confirms the reported higher R1 and R2 relaxivities of gadobenate relative to gadopentetate, gadodiamide, gadoversetamide, and gadoteridol. These differences were present at both 1.5T and 3T. These data help explain the potential clinical benefits of higher relaxivity agents and confirm the results seen in the human in vivo crossover trials already reported.12–15 These results also support the prediction that higher R2 values would provide similar advantages over lower R2 relaxivity agents for PWI per administered dose/volume of GBMCA and would yield higher CNR and/or shorter scanning times for similar diagnostic CNR end points.
Footnotes
This work was supported by a grant of Seronorm and a grant to support data entry provided by Bracco Diagnostics.
Paper previously presented in part at: Annual Meeting of the American Society of Neuroradiology, May 6–12, 2006; San Diego, Calif.
References
- Received August 24, 2007.
- Accepted after revision October 6, 2007.
- Copyright © American Society of Neuroradiology