Abstract
BACKGROUND AND PURPOSE: Defects at the skull base leading to spontaneous CSF rhinorrhea are rare lesions. The purpose of our study was to correlate CT and MR findings regarding the location and content of CSF leaks in 27 patients with endoscopic sinus surgery observations.
MATERIALS AND METHODS: Imaging studies in 27 patients with intermittent CSF rhinorrhea (CT in every patient including 10 examinations with intrathecal contrast, plain CT in 2 patients, and MR in 15 patients) were analyzed and were retrospectively blinded to intraoperative findings.
RESULTS: CT depicted a small endoscopy-confirmed osseous defect in 3 different locations: 1) within the ethmoid in 15 instances (53.6% of defects) most commonly at the level of the anterior ethmoid artery (8/15); 2) adjacent to the inferolateral recess of the sphenoid sinus in 7 patients including one patient with bilateral lesions (8/28 defects, 28.6%); 3) within the midline sphenoid sinus in 5 of 28 instances (17.9%). Lateral sphenoid defects (3.5 ± 0.80 mm) were larger than those in ethmoid (2.7 ± 0.77 mm, P ≤ 0.029) or midsphenoid location (2.4 ± 0.65 mm, P ≤ 0.026). With endoscopy proven arachnoid herniation in 24 instances as reference, MR was correct in 14 of 15 instances (93.3%), CT cisternography in 5 of 8 instances (62.5%). Plain CT in 1 patient was negative.
CONCLUSION: In patients with a history of spontaneous CSF rhinorrhea, CT was required to detect osseous defects at specific sites of predilection. MR enabled differentiating the contents of herniated tissue and allowed identification of arachnoid tissue as a previously hardly recognized imaging finding.
The term “spontaneous” CSF rhinorrhea has been applied to describe nasal discharge of CSF unrelated to trauma, surgery, malformation, tumor, or previous radiation therapy.1–4 Spontaneous CSF rhinorrhea is uncommon. Estimates of the spontaneous cause among all causes of CSF rhinorrhea are subject to variation ranging from only 6%,5 11.4%,6 14%,3 21%,7 to 23%.8 Periodic release of CSF from the nose was first described by Galen in 200 B.C. and was considered a physiologic phenomenon until Thomson, in 1899, assembled 21 patients in a monograph reporting spontaneous CSF rhinorrhea as a pathologic clinical entity.9,10
Spontaneous CSF rhinorrhea has been recognized as a distinct entity with respect to clinical presentation,2,11,12 treatment,13–15 and propensity for recurrence.8,16,17 As early as 1968, Ommaya et al9 postulated the existence of “high-pressure leaks” related to intracranial tumors and of “normal pressure leaks” occasionally associated with empty sella. The role of empty sella as an indicator of raised intracranial pressure as well was supported by the observation of elevated CSF pressure in individual patients11 and in a series of 10 patients who underwent lumbar puncture after sealing of the defect.18 In addition to the presence of an empty sella as a radiologic sign,19 a common clinical constellation in patients with spontaneous CSF rhinorrhea is female sex, middle age, and obesity.8,14,15,18–22
Spontaneous CSF leaks have been postulated to represent a manifestation of benign intracranial hypertension22 or pseudotumor cerebri.23 Pulsatile-increased hydrostatic pressure is capable of bone erosion during the course of many years.2,24 To become effective as a CSF leak, bone erosion and creation of an osteodural defect is required to occur at pneumatized parts of the skull base leading to communication of the subarachnoid space with the sinonasal spaces or temporal bone cavity. Related to CSF rhinorrhea, a review of the literature up to 197210 identified the cribriform plate, craniopharyngeal canal, sella, and spheno-occipital synchondrosis as possible sites of predilection. Arachnoid granulations in proximity to the ethmoid and sphenoid sinus have been implicated as precursors of osteodural leaks.2 Accordingly, arachnoid granulations causing erosion of the temporal bone may present with CSF otorrhea.2,25,26
Among the imaging techniques used to localize the site of the fistula, radionuclide isotope cisternography and CT cisternography were of limited sensitivity in 66% of patients only.3 When active leaks were present, CT cisternography provided positive results in 85% of patients.27 However, in cases of inactive fistulas, CT cisternography failed to recognize the site of leakage in 27.7%28 and in 19% of patients.29 Advances in CT and MR imaging techniques have improved sensitivity, which amounted to 88.25%30 and 93%31 for high-resolution CT and for MR cisternography to 89%,6,31 93.6%,28 and 100%32,33 even in patients with inactive leaks. Therefore, high-resolution CT, MR cisternography, or a combination of both techniques have replaced the previously used invasive procedures.
A confounding nomenclature exists regarding the contents of osteodural defects such as meningocele,10,14 meningoencephalocele,4 encephalocele,11,34 meningeal or arachnoid hernia,24,35 arachnoid diverticulum,36 or arachnoid cyst.37 These differing designations reflect variable contents of herniation and occasional inaccuracy because of the limited ability to visualize the lesions by imaging24,29 and during transcranial surgery.1,10 Knowledge of the contents of herniation may modify the grafting technique and therefore facilitates preoperative planning.16 The endoscopic skull base approach has rendered direct visualization of the defect and its contents feasible.38–40 Therefore, endoscopy was chosen as a standard of reference in this study. CT and MR findings in this series of patients with spontaneous CSF rhinorrhea were particularly assessed regarding the contents of herniation and location and correlated with endoscopy. Predisposing factors (arachnoid granulation, empty sella) and the size of the osseous defect were assessed on CT images.
Patients and Methods
The imaging findings in 27 consecutive patients with spontaneous intermittent, nontraumatic CSF rhinorrhea were retrospectively analyzed. The presence of CSF rhinorrhea and preoperative imaging by CT, CT cisternography, or MR imaging focused on the skull base were the selection criteria for the entire series. The review included the location, size, and content of the leaks, and additional findings and was conducted by the neuroradiologist (B.S.) blinded to the observations during endoscopic sinus surgery. The imaging findings were correlated with the observations during endoscopic sinus surgery.
Patients with a history of trauma, previous paranasal sinus or skull base surgery, and congenital malformation of the skull base were excluded from the study. Because correlation with endoscopic findings was the standard of reference for location and content, CSF leaks related to the petrous bone were not included.
CSF rhinorrhea had been proved by a positive β2 transferrin test in all patients. Every patient had undergone endoscopic sinus surgery by the same ear, nose, and throat surgeons (D.S. and D.H.) between 1993 and 2006. Since 1997, the patients were operated on with intrathecal application of fluorescein for confirmation of the site of the leak. The location of the defect and concomitant intraoperative findings had been documented as digital images and on video with DVD. Histologic examination had been obtained in every patient. The clinical records were reviewed for follow-up visits to assess potential recurrence of CSF rhinorrhea.
Before 1997, imaging consisted of paranasal sinus plain CT in the axial and coronal plane in every patient with a field of view (FOV) of 120 mm, section thickness of 1 mm, and matrix of 512 × 512. Coronal plain CT was diagnostic in 2 patients. In 10 patients in whom plain CT was not considered diagnostic, CT cisternography was performed. With a section collimation of 1 mm and with use of the same parameters, CT cisternography was performed in the coronal plane after lumbar intrathecal contrast administration (10 mL iopamidol 240 mg I/mL). From 1997 on, plain CT was obtained as a spiral dataset in the axial plane with 1-mm collimation, and matrix size was 1024 × 1024. Section reconstruction was 1.25 mm of section thickness, in 0.7-mm increments. Multiplanar reconstruction images were obtained in the axial and coronal planes with 1 to 2 mm of thickness; image analysis was based on CT cisternography and plain CT first. Measurement of the size of the osseous defect was performed on axial and coronal images. The maximum dimension of the defect was recorded. To assess differences in the sizes among the locations of the ethmoid, midsphenoid, and lateral sphenoid, we analyzed the data using the unpaired Student t test, with a significance set at P ≤ .05.
Assessment of the MR Images Followed Previous Analysis of the CT Examination
Heavily T2-weighted coronal MR images (MR cisternography) replaced intrathecal contrast administration in 15 patients. MR images were performed on a 1.5T scanner with an 8-channel phased array head coil. The sequence used was a constructive interference in steady state (CISS). Imaging parameters for coronal images were TR, 11.6; TE, 5.8 ms; NEX, 2; section thickness, 0.8 mm; matrix, 256 × 256; and FOV, 120 mm. Imaging parameters for axial and sagittal T2 fast spin-echo images were TR, 4000 to 4200 ms; TE, 90 ms; NEX, 3; section thickness, 3-mm thick interleaved contiguous sections; matrix, 448 × 224; echo-train length, 13; and FOV, 200 mm. Parameters for T1 coronal and axial images were TR, 400 to 450 ms and TE, 10 to 14 ms before and after intravenous contrast administration. CT and MR had been performed within 2 days, in most of the patients on the same day.
Results
Clinical Findings
A total of 27 patients (17 female, 10 male) had undergone endoscopic sinus surgery between 1993 and 2006. The average age was 51.1 years with an age range from 17 to 74 years. The patients were investigated because of spontaneous, intermittent CSF rhinorrhea that had been present for 1 month to 5 years, on average for 10.2 months (On-line Table). A history of meningitis was positive in 5 patients. After endoscopic sealing of the defect, no recurrence of CSF rhinorrhea was found after an average follow-up of 5.5 years for 22 patients. In 2 patients, recurrence was observed within 8 months and was treated again. These patients have been free of symptoms for 7 and 3.5 years, respectively. In 3 patients, the follow-up period was between 5 and 9 months.
Imaging Findings
The imaging findings are summarized in the accompanying On-line Table. CT depicted 28 osseous defects: on the right in 15 patients and on the left in 13. The CSF leaks were related to the ethmoid in 15 (53.6%) patients and affected the sphenoid sinus with 13 (46.4%) defects. The lateral wall of the sphenoid sinus was the site of 8 (28.6%) defects in 7 patients, including 1 patient with bilateral lesions. The midline sphenoid was affected in 5 (17.9%) of 28 leaks.
Ethmoid Location.
In 11 of 15 instances, high-resolution bone algorithm CT images delineated a small osteodural interruption along the cribriform plate leading into the olfactory cleft with a small soft tissue lesion (Fig 1). In 2 patients, the leak was via the lateral lamella into the anterior ethmoid cells and, in another 2 patients, at the junction of the cribriform plate with the lateral lamella and fovea ethmoidalis, leading into the frontal recess (Fig 2) and anterior ethmoid, respectively (On-line Table).
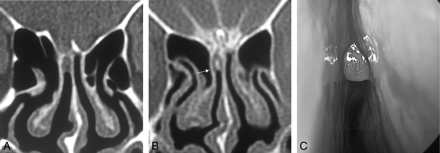
A–C, A 52-year-old man (patient 2) with intermittent CSF rhinorrhea for 2 months. A coronal high-resolution bone window noncontrast CT (A) early in this series reveals a soft tissue lesion within the right olfactory cleft adjacent to an osseous defect at the cribriform plate. After intrathecal administration of contrast material (B), a small loculation of contrast material is visualized (arrow) surrounded by isoattenuation of soft tissue. Endoscopic surgery revealed an arachnoid lined pouch within the olfactory cleft (C).
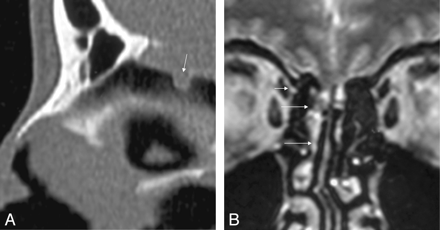
A, B, Sagittal high-resolution bone window image depicts a 2-mm defect within the cribriform plate (arrow) in a 30-year-old patient with 4 months of intermittent CSF rhinorrhea (patient 8). The coronal T2-weighted image reveals a normal olfactory cleft. Isointense arachnoid tissue and CSF loculation originate from the junction of the cribriform plate and lamella lateralis and herniate into the frontal recess (long arrow) down to the middle meatus (long arrow). Proximity of the osteodural defect to the entrance point of the ethmoid artery is shown (short arrow).
There were 8 (53.3%) of 15 lesions related to the ethmoid along the course of the anterior ethmoid artery (Figs 2, 3). The defect was more anterior along the cribriform plate in 5 patients and just posterior to the anterior ethmoid artery in 2 patients. The mean size of the lesions was 2.77 ± 0.77 mm.
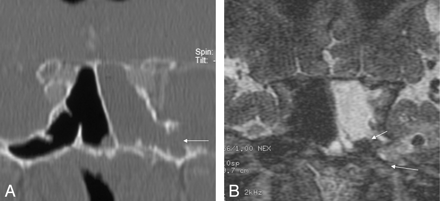
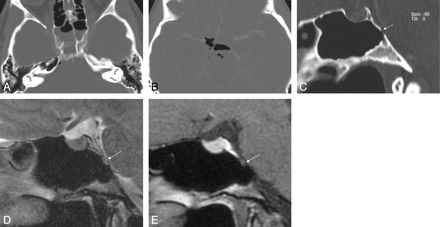
A, B, Coronal high-resolution bone window CT image (A) depicts a small erosion of the inferolateral recess (arrow) in a 70-year-old woman (patient 18) with massive intermittent CSF rhinorrhea for 7 months. The coronal T2-weighted MR image (B) shows a fluid level within the left sphenoid sinus, air within ventricles, and an air bubble below the left inferior temporal gyrus. Adjacent subcortical gliotic changes are present. A lesion with soft tissue isointense components and CSF is shown (long arrow), which corresponded to endoscopy, and histologic examination proved the presence of arachnoid tissue.
CT cisternography revealed a contrast-filled small pouch in 4 of 6 patients (Fig 1). Plain CT only was performed in 1 patient. In this patient, arachnoid and mucosal tissue could not be distinguished. T2-weighted MR images performed in 8 patients revealed extracranial extension of CSF into a small pouch located within the olfactory cleft in 6 patients and within the ethmoid cells in 2 patients. Arachnoid herniation was indicated by soft tissue isointense strands of tissue intermingled with CSF signal intensity (Fig 2B). Concomitant brain changes were present in 2 patients only. The medial orbital gyrus reached down to the level of the cribriform plate in 1 patient; focal gliosis of the gyrus rectus was present in another patient.
Lateral Sphenoid Location.
The defect was lateral to the foramen rotundum within the anterior portion of the inferolateral recess in 8 instances (Figs 3, 4). Rounded pits with scalloped margins caused by prominent arachnoid granulations were found on CT in 7 of 8 defects in 6 patients, including 1 patient with bilateral leakage. Arachnoid granulations without leakage were present in 2 of the remaining 5 patients on the opposite side as well. With an average size of 3.5 ± 0.80 mm, the osseous defects in the lateral sphenoid wall were significantly larger (P ≤ .026) than those in the midsphenoid (2.4 ± 0.65 mm) and with P ≤ .029 significantly larger than those in the ethmoid location (2.77 ± 0.77 mm) as well.
A–D, An 18-year-old man with 1 episode of CSF rhinorrhea (patient 22). CT images depict arachnoid pits lateral to sphenoid sinus and osseous erosion lateral to the foramen rotundum (short arrow). The coronal T2-weighted image (C) reveals a CSF-filled pouch extending into the right inferolateral recess with some arachnoid strands (arrow). The endoscopic image (D, view from medial) depicts an arachnoid pouch (arrow) bulging into the sinus lumen.
MR delineated CSF signal intensity with soft tissue isointense components in small lesions (Fig 3), and CSF filled pouches mixed with isointense arachnoid strands in larger lesions (Fig 4) arising from the inferolateral recess in 6 instances. The lesions were in continuation with the CSF space of the middle cranial fossa. Focal inferior temporal gyrus gliosis and pneumocephalus were present in 1 patient at the time of MR imaging (Fig 3). Minor contrast enhancement was present at the periphery of the arachnoid pouches in 3 patients.
Midline-Sphenoid Location.
Of 5 patients, CT depicted defects within the sphenoid roof in 3 cases. In another 2 patients, the defect was within the posterior wall (Fig 5). A contrast-filled sinus indicating active CSF leakage was found after CT cisternography in 2 cases and a fluid-filled sinus on MR cisternography in 1 patient. Inexperience with this location and small size (average, 2.4 mm) had caused the midsphenoid leaks to be overlooked on the initial reading before this study in 3 of 5 cases.
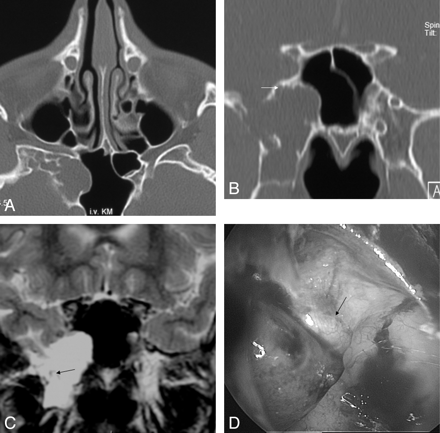
A–E, A 38-year-old woman (patient 24) with a history of meningitis 2 months ago. After sudden onset of a headache, the axial CT (A, B) shows a fluid level within the sphenoid sinus and subarachnoid air in the right prepontine and suprasellar cistern. At 14 days later after referral for further evaluation, sagittal CT (C) shows an aerated sinus and a small osseous defect within the posterior wall of the sphenoid sinus (arrow). Sagittal T2 and T1-weighted MR images (D, E) depict a small lesion (arrow) that herniates into the sphenoid sinus. Only in the clinical setting of CSF rhinorrhea the lesion is suspected to correspond to arachnoid herniation.
Empty Sella.
An empty sella and partially empty sella was found in 11 (40.7%) and 6 (22.2%) patients, respectively. The prevalence was 40% for 10 male patients and 76% in 17 female patients compared with 63% for the entire series.
Intraoperative Endoscopy Findings.
The CSF leak treated by endoscopic sinus surgery corresponded to the location recognized by CT and MR imaging in all of the 28 locations. During endoscopic sinus surgery, arachnoid herniation was observed in 24 of 28 osteodural defects. Endoscopy confirmed the presence of prominent surface arachnoid vessels in 3 lateral sphenoid herniations, which presented with minor contrast enhancement on MR imaging. Treatment consisted of a fascia underlay and a free extradural mucosa graft in 15 patients with ethmoid leakage. In 12 patients with defects related to the sphenoid, the approach was transnasal as well. The arachnoid hernia was coagulated, and the defect was plugged by a fascial underlay and an extracranial free mucosal graft overlay as well.
Discussion
Nontraumatic spontaneous, direct CSF rhinorrhea may arise from defects in the skull base related to the anterior, middle, and posterior cranial fossae and the sella turcica. Leaks in these locations cause direct CSF rhinorrhea. Indirect CSF rhinorrhea may originate from defects related to the petrous bone and appears as otorhinorrhea when the Eustachian tube directs CSF flow into the nasal cavity via the nasopharynx.2,25,41 Both manifestations imply an osteodural defect that creates a communication of the subarachnoid space with the sinonasal spaces or middle ear cavity.
Increasing attention has altered recognition of CSF dynamics as an important pathogenic factor. In an earlier report on 45 patients with posttraumatic and spontaneous CSF fistulas, Shetty et al31 state that “none of the patients with spontaneous CSF fistulas had raised intracranial pressure.” In a later study on a subgroup of 11 patients with spontaneous sphenoid sinus fistulas, the authors concluded that the constellation of empty sella, arachnoid pits, and extensive pneumatization plays a role in the pathogenesis of sphenoid sinus fistulas. The prevalence of empty sella was 63%.4 In our series, 63% of patients harbored an empty or partially empty sella as well. No slit ventricles, tonsillar ectopia, ectasia of the optic nerve sheaths, or meningeal enhancement were found as potential additional indicators of altered intracranial pressure in this series. Mild hydrocephalus was present in 1 patient aged 70 years, and 1 patient harbored an occipital arteriovenous malformation. Increased venous pressure caused by venous sinus stenosis has been described, leading to pseudotumor cerebri and anterior cranial fossa spontaneous CSF leakage.23
Arachnoid granulations related to the temporal bone2,25 and the ethmoid and sphenoid sinus in 1 patient each2 have been implicated as the responsible lesions for spontaneous CSF otorrhea and rhinorrhea.
A congenital cause of the skull base defects as alternatively proposed by Ommaya et al9 is unlikely as pneumatization of the ethmoid and sphenoid sinus does not occur until later childhood. The average age of manifestation with 49.6 years in 16 patients21 and 51 years in our series are in favor of an acquired cause. Contrary to true meningocele or meningoencephalocele concomitant skull base and brain malformation are not found.4,24 The frequently small size with an average diameter of 2.87 ± 0.84 mm and occasional multiplicity of osteodural leaks, as in one of our patients with sphenoid sinus involvement, are further indicators of acquired defects.
The development of osteodural defects is bound to specific sites only and probably is caused by interaction of CSF pulsation with the skull base. The anatomic disposition differs between the anterior, middle, and posterior cranial fossa.
The cribriform plate is the most common site of fistulas in spontaneous cases.3,31,42 Fistulas localized by endoscopic surgery14 affected the cribriform plate in 6 (28.6%) of 21 patients and were disclosed by MR cisternography at the same location in 12 (32%) patients reported by Shetty et al.31 In this series, 11 (39.3%) of 28 defects affected the cribriform plate. The lamella lateralis followed in frequency in 4 (14.3%) patients in our study, 18% in the series by Shetty,31 and 28.6% of patients investigated by Lopatin et al.14
In this series, MR and CT cisternography disclosed arachnoid herniation in 12 of 15 patients with leaks related to the ethmoid. Endoscopic surgery proved arachnoid herniation in 13 of 15 patients; in 1 patient, noncontrast CT had been unable to differentiate the soft tissue lesion from mucosal swelling. The occurrence of osteodural leaks along the course of the anterior ethmoid artery in 8 of 15 patients indicates reduced skull base resistance at this location, which previously has been noticed as a common site for posttraumatic CSF fistulas only.43 Recognition of the course of the anterior ethmoid artery on imaging is crucial to avoid complications during endoscopic treatment.44
As far as imaging is concerned, arachnoid herniation has hardly been recognized as an important finding in conjunction with osteodural defects.4,37,45 In those patients examined by MR in this study, pouches of CSF intensity with strands of soft tissue were arising from the osteodural defects; contrast enhancement was not present in ethmoid lesions. Probably indicated as “meningocele” in most instances on imaging,14,15,21 this term is imprecise because fenestration of the dura overlying the osseous defect is a frequent finding during surgery.25,29,46 Minutoli et al35 reported a 63-year-old man with “small arachnoid hernia in the left nasal cavity between the nasal septum and middle turbinate.” As early as 1987, MR contributed to recognition of a similar lesion within the ethmoid designated as “arachnoid cyst.”37
In an anatomic study,47 dural dehiscences and arachnoid sleeves that accompany the olfactory fila to the olfactory cleft were found as predisposing factors in this location. Accordingly, surgical reports describe a “vascularized sleeve of arachnoid passing through a small hole in the left cribriform plate” in 2 cases9 and “arachnoid covered tissue” extending into the frontal recess in 1 patient with pseudotumor cerebri.48 Arachnoid granulations, which have been noticed to be related to the ethmoid in 1 patient previously,2 were present in 1 of 15 patients in this series only. In the remainder, neither anterior nor middle cranial fossa arachnoid pits were found. As indirect evidence of a CSF fistula, a low-lying rectus gyrus has been reported in 14 (52%) of 27 patients with traumatic and spontaneous fistulas related to the ethmoid.31 This was the case in one of our patients only.
The sphenoid sinus was the second most frequent site of spontaneous osteodural defects. The lateral sphenoid sinus wall and the midline sphenoid were affected with 8 (28.6%) of 28 lesions and with 5 (17.8%) defects, respectively. A similar distribution was reported by Schlosser and Bolger.11 Of a total of 22 leaks, the lateral sphenoid sinus wall was affected in 36.4%, the central sphenoid in 18.2%. In an anatomic study on 276 half-sphenoid bones examined by Hooper,49 9.8% of lateral sphenoid bones harbored osseous defects. The location corresponded to the endocranial surface of the lateral craniopharyngeal canal in 14 of 27 defects. The remainder occurred along the course of the internal carotid artery. Originally described by Cruveilhier in 1877 and Sternberg in 1888,50 the lateral craniopharyngeal canal corresponds to a fusion line of the basisphenoid with the greater sphenoid wing.51 Persistence of this canal into adulthood was found in 4% of patients.50 Probably more relevant are aberrant arachnoid granulations, which have penetrated the dura but failed to reach a venous sinus in this location.2 Accordingly, a smooth lobulated appearance of the defects has been described from an anatomic standpoint49 as well as by CT and has been designated as “arachnoid pits.”4 A corresponding appearance was found on CT images in 7 (87.5%) of 8 defects in this series. Bilateral arachnoid granulations were associated with bilateral osteodural breaches in 1 patient, with a unilateral defect in 2 patients. Bilateral lesions leading to CSF leakage, as in one of our patients, were previously reported in 1 of 11 patients.4 The concept of arachnoid granulations as precursors of osteodural defects seems to hold true for the lateral wall of the sphenoid sinus.
Apart from the presence of arachnoid granulations in the position of the so-called Sternberg canal, pneumatization of the inferolateral recess below the middle cranial fossa is a prerequisite for a CSF leak to occur. In an anatomic investigation52 as well as a CT study,4 extension of the sphenoid sinus lateral to the foramen rotundum was noted in 40% and 23% of normal sinuses, respectively. Therefore, erosion of bone by arachnoid granulations is only significant when it affects a pneumatized part of the skull. This predisposition occasionally is termed congenital51 and has been implicated as a cause of metachronous CSF rhinorrhea arising from the lateral wall in 2 siblings.53
MR cisternography complements the high-resolution CT examination regarding the content of herniation through the osteodural defect. Extension of arachnoid strands from the middle cranial fossa into the sphenoid sinus was present in 7 of 8 defects resulting in formation of arachnoid diverticula. An air-fluid level was found in 2 cases with active leakage, in conjunction with pneumocephalus in 1 patient. A gliotic area was present adjacent to a large air bubble in this patient and in another patient with a defect related to the anterior ethmoid. It is unclear whether these changes in subcortical location were related to previous inflammation. Large distance to the neck of the arachnoid herniation and lack of any concomitant changes of the cortex make previous intermittent brain herniation unlikely as well as occult trauma.
Brain herniation was neither found intraoperatively nor on imaging in any of the 8 lesions originating from the lateral wall of the sphenoid sinus. Only in 1 leak (of 28 defects), arachnoid herniation and gliotic brain tissue were hard to distinguish during surgery, but histologic examination unequivocally disclosed arachnoid tissue only. This is contrary to findings on MR cisternography in 11 patients published by Shetty et al.4 Herniation was reported to be present in the arachnoid in 2 patients and brain tissue in 9 patients. In 7 of 9 patients, however, the lesions were described to arise from arachnoid pits, which make arachnoid herniation more likely than encephalocele. In 1 patient with bilateral “meningoencephaloceles,” the findings were commented, that “the dura arachnoid complex had given way to CSF leakage.” A markedly larger size of the defect in 1 illustrated example, however, would explain the difference in findings at least for this patient.
Minor contrast enhancement was present in 3 of 8 lesions related to the inferolateral recess in this series. Contrast enhancement did not correlate with inflammation and did not correspond to surface mucosa on histologic examination. Despite the fact that during endoscopic surgery minute arachnoid vessels always were visible along with arachnoid herniation, the surface vessels were much more prominent in these patients. We assume that this phenomenon is related to venous congestion caused by narrowing at the neck of the arachnoid diverticulum.
In the literature, the entire spectrum of contents of herniation has been described during surgery including meningocele,14 arachnoid tissue,4,54 or encephalocele.34,54 On rare occasions, osteodural defects of the lateral sphenoid sinus did not cause herniation of any tissue at all.46
Sound principles regarding endoscopic surgery of CSF leaks consist of adequate exposure, preparation of the fistula, and adequate grafting.16 Before grafting, differentiation of the contents of herniation is essential. As grafting material, the use of bone or cartilage is not required unless herniation of the meninges or brain is present.16 Therefore, knowledge of the contents of herniation before endoscopic surgery has implications on treatment planning.
The midline sphenoid sinus was the third most common location with 5 (17.8%) of 28 defects recognized in this location. The roof was affected anterior to the tuberculum sellae in 3 of our patients and the posterior wall in 2 patients. Osteodural defects related to the sphenoid roof are rare. However, 2 patients with defects in the paramedian sphenoid sinus location have been reported by Silva and coworkers.43 Lopatin and coworkers14 describe a midline fistula site in 4 of 9 patients with a sphenoid sinus location of spontaneous CSF leaks. During endoscopic surgery, leaks presented with arachnoid herniation as demonstrated in Figs 1C and 4D in our series. Arachnoid herniation has previously been described in individual patients to arise from the sellar floor itself in association with empty sella.9,36,55 In a series of 38 patients who were assumed to harbor a trans-sellar fistula, the authors admit that “definitive determination of the location of the fistula was often difficult.” On follow-up, 33% to 50% of their patients experienced recurrence after surgery or shunt placement.56 It is not unlikely that the true location of the fistula escaped detection. Our findings conform to observations by Shetty and coworkers.4 In a retrospective analysis of 11 patients with spontaneous sphenoid sinus fistula, none of the patients showed evidence of the CSF leak from the sellar floor.
The posterior wall of the sphenoid sinus is a rare location of osteodural defects. Obrador10,57 postulates the spheno-occipital synchondrosis as a predilection site. Two of our patients and 1 case reported by Bonfils et al58 corresponded to this location. An alternative hypothesis is weakening of the posterior wall of the sphenoid sinus by remnants of the primitive notochord. Macdonald et al59 described 1 patient with ecchordosis physaliphora arising from the remains of the notochord as a cause of posterior fossa dural erosion and spontaneous CSF rhinorrhea.
Bilaterality and multiplicity of osteodural defects have been implicated as support for a spontaneous nontraumatic cause of osteodural defects.2,4,24 Arachnoid granulations that did not find a venous termination are considered an important anatomic disposition2 and, in this series, were confirmed for the lateral sphenoid sinus location. Bilaterality of CSF leaks may occur in isolated cases4 and is explained by the frequent symmetric distribution of arachnoid villi.2 In every patient who undergoes imaging evaluation for a CSF leak, potential multiplicity of defects has to be borne in mind. Schlosser and Bolger21 recognized multiple simultaneous skull base defects during surgery in 5 (31.25%) of 16 patients with nasal CSF leaks. Raghavan et al60 report 1 patient adding 5 cases from the literature with an anterior cranial fossa CSF fistula and an additional leak located at the petrous bone. In the search for osteodural defects, the petrous bone should therefore be included into the initial diagnostic CT evaluation.2,16,26,33
The petrous apex itself may be an additional location for cystic lesions with CSF signal intensity. Lesions reported as petrous apex “arachnoid cyst”61 and petrous apex “cephalocele”62 reflect varying designations for the contents of herniation in this location as well. In 3 of 10 patients with a petrous apex “cephalocele,” surgical confirmation was obtained.62 Histologic examination revealed a meningocele in 2 instances and an arachnoid cyst in 1 patient.
Despite its accuracy in delineating the location and contents of osteodural defects, the imaging findings reported are limited to patients in whom CSF rhinorrhea is present. Because the selection criterion was positive CSF rhinorrhea, specificity of the findings therefore cannot be addressed. However, in view of the literature, our findings are representative because incidental osteodural defects have not been encountered with 1 exception only. In a 34-year-old woman investigated for a headache, CT and MR revealed a lesion within the sphenoid sinus interpreted as a mucocele.45 Surgery disclosed arachnoid herniation originating from an osteodural defect that had passed unrecognized preoperatively.
Conclusion
High-resolution CT imaging is required to recognize osteodural leaks at specific sites of predilection along the ethmoid, midline sphenoid, and lateral sphenoid sinus and to delineate the anatomy for the endoscopic surgical approach. Anatomic dispositions differ for the 3 principal locations and consist of arachnoid granulations for lateral sphenoid sinus lesions (7 [87.5%] of 8 lesions), of osteodural interruption at the cribriform plate in conjunction with the olfactory fila in 11 (73.3%) of 15 lesions, and are undetermined yet for the midline sphenoid location. Leaks related to arachnoid granulations along the lateral sphenoid were significantly larger than those along the ethmoid and midline sphenoid osteodural defects. MR imaging supplements the CT examination with respect to recognition of the presence of herniated tissue and differentiation of its contents. Arachnoid herniation was correctly predicted by MR imaging in 14 (93.3%) of 15 instances.
Footnotes
Indicates article with supplemental on-line tables.
References
- Received February 5, 2006.
- Accepted after revision August 18, 2007.
- Copyright © American Society of Neuroradiology