Abstract
BACKGROUND AND PURPOSE: Results of endovascular treatment of PICA aneurysms are not well established. The purpose of this study was to report incidence, clinical presentation, and outcome of endovascular treatment in 46 patients with 47 posterior inferior cerebellar artery (PICA) aneurysms.
MATERIALS AND METHODS: Of 2169 aneurysms treated between January 1995 and March 2007, 60 were located on the PICA (incidence, 2.8%). Forty-seven proximal PICA aneurysms in 46 patients were treated with endovascular techniques, 37 ruptured (79%) and 10 unruptured (21%). Four patients presented with lower cranial nerve palsies. Mean aneurysm size was 6.8 mm (median, 6 mm; range, 2–32 mm). Forty-three aneurysms were occluded with coils (6 including the PICA origin), and 4 were treated with proximal vertebral artery (VA) occlusion.
RESULTS: Four aneurysms treated with proximal VA occlusion were not occluded. Procedural rupture occurred in 9 aneurysms leading to death in 2 patients and to permanent disability in 1 patient. One patient developed lateral medullary and cerebellar infarctions after PICA occlusion. Combined mortality and morbidity was 8.6% (4 of 46). Outcome at 6 months in 38 surviving patients was good in 35 and moderate in 3. No hemorrhage occurred during 109 patient-years of follow-up. Symptoms of mass effect resolved in all 4 patients.
CONCLUSION: In our experience, PICA aneurysms were challenging lesions, prone to procedural rupture. In some instances, endovascular treatment required occlusion of the parent PICA; usually this was well tolerated. In other instances, treatment required occlusion of the VA. Although this was effective in alleviation of symptoms of mass effect, it was not effective in causing thrombosis of the aneurysm.
Aneurysms of the posterior inferior cerebellar artery (PICA) are rare. Surgery for these aneurysms is challenging due to the deep location and intimate relation with the medulla and cranial nerves IX, X, and XI.1–8 Although endovascular treatment of intracranial aneurysms is increasingly used as an alternative to surgery, endovascular results of PICA aneurysms are not well established.9–12 In this study, we report our experience with endovascular treatment of 47 proximal PICA aneurysms in 46 patients.
Patients and Methods
Patients
Between January 1995 and March 2007, 2169 aneurysms were treated in our institution, 940 surgically and 1229 endovascularly. Sixty aneurysms in 58 patients were located on the PICA. Incidence of PICA aneurysms was 2.8% (60 of 2169) of all treated aneurysms and 18% (60 of 334) of posterior circulation aneurysms. Since the beginning of the study period, endovascular treatment was the therapy of choice for posterior circulation aneurysms and only 7 of 334 posterior circulation aneurysms were treated surgically, 5 of which were located on the PICA. Of 55 PICA aneurysms that were treated endovascularly, 8 aneurysms in 7 patients were located distally on the PICA, and these aneurysms were excluded from analysis. The present study group consists of 47 (proximal) PICA aneurysms in 46 patients treated with endovascular techniques. Patient and treatment characteristics of 37 ruptured PICA aneurysms (79%) are summarized in on-line Table 1 and of 10 unruptured PICA aneurysms (21%), in on-line Table 2. Of 10 unruptured aneurysms, 2 were incidentally found on imaging unrelated to presenting symptoms, 4 were additional to another ruptured aneurysm, and 4 presented with symptoms of mass effect on the medulla, causing palsies of cranial nerves V, VI, or VII (Fig 1).
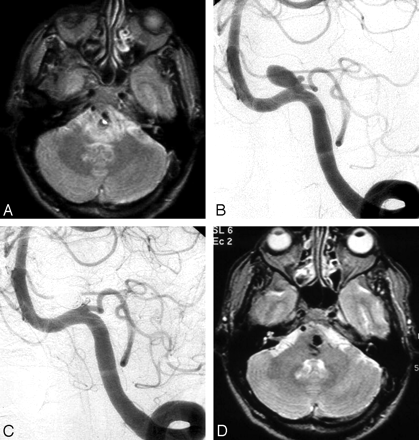
A 49-year-old man presenting with left abducens and right facial nerve palsies. A, MR imaging demonstrates an aneurysm compressing the medulla and pons with surrounding edema. B, Left vertebral angiogram shows a PICA aneurysm. C, After coiling, complete aneurysm occlusion is seen. D, MR imaging 6 months later shows regression of edema; cranial nerve palsies are cured.
There were 8 men (17%) and 38 women (83%) with a mean age of 54.7 years (median, 53 years; range, 24–83 years). Clinical grading according to the Hunt and Hess (HH) scale at the time of treatment in 37 patients with ruptured PICA aneurysms was the following: HH I–II in 20 patients, HH III in 4 patients, and HH IV–V in 13 patients. Timing of treatment after subarachnoid hemorrhage (SAH) was 0–3 days in 18 patients, between 4 and 14 days in 13 patients, and ≥14 days in 6 patients. Mean aneurysm size was 6.8 mm (median, 6 mm; range, 2–32 mm). There were 42 small aneurysms (≤10 mm), 4 large aneurysms (11–25 mm), and 1 giant aneurysm (32 mm).
Endovascular Treatment
Indication for and type of endovascular treatment was a joint decision between interventional neuroradiologists and neurosurgeons in patients presenting with acute or delayed SAH both in a delayed time course or with unruptured aneurysms. In patients with PICA aneurysms with favorable geometry, selective coiling was the preferred treatment. In patients with aneurysms with the PICA originating from the sac or with a wide neck based on the PICA, treatment could consist of selective coil occlusion of the aneurysmal sac sparing the PICA (Fig 2), coil occlusion of the aneurysmal sac with deliberate occlusion of the PICA (internal coil trapping in on-line Table 1) (Fig 3), coil occlusion of the aneurysmal sac including the PICA origin and vertebral artery (VA) (internal coil trapping plus VA in on-line Table 1) (Fig 4), and stent-assisted coiling and vertebral coil or balloon occlusion proximal to the PICA (Fig 5). The type of treatment was mainly dependent on clinical presentation (acute or delayed SAH, mass effect, or incidental finding). Deconstructive treatments with deliberate PICA origin occlusion were generally performed in patients in poor clinical grades after acute SAH not amenable for acute surgery only. In cases with deliberate proximal PICA occlusion, we anticipated the risk of ischemia in the PICA territory. However, most patients do not develop infarctions after PICA occlusion; when infarctions do occur, risk of permanent morbidity is fairly low.13,14
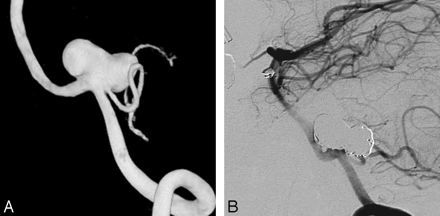
A 52-year-old woman with an incidentally found PICA aneurysm. A, 3D left vertebral angiogram demonstrates PICA aneurysm with the PICA originating from the sac. B, Six-month follow-up angiogram shows complete occlusion with preservation of flow in the PICA.
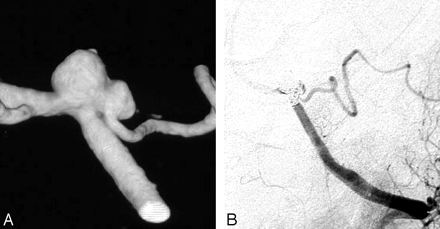
Ruptured PICA aneurysm in a 62-year-old man presenting in poor clinical condition. A, 3D right vertebral angiogram shows a wide-necked PICA aneurysm. This aneurysm was treated with coil occlusion, including the parent PICA (internal trapping). B, CT scan 6 months later demonstrates infarction in the PICA territory; however, the patient was asymptomatic.
Acutely ruptured PICA aneurysm in a 46-year-old woman. A, 3D left vertebral angiogram shows PICA aneurysm broad-based on the VA and PICA, originating from the sac. B, Angiography in the process of coiling, with occlusion of the PICA including the VA and PICA origin. MR imaging 3 months later (not shown) revealed small bilateral peripheral asymptomatic cerebellar infarctions, probably as a result of vasospasm.
A 68-year-old woman presenting 18 days after SAH from PICA aneurysm. A, Right vertebral angiogram demonstrates PICA aneurysm with PICA originating from the sac. B, Left vertebral angiogram after coil occlusion of the right VA proximal to the PICA (arrow) demonstrates persistent opacification of the aneurysm. MR imaging 6 months later (not shown) was equivocal for aneurysm thrombosis; there was no recurrent hemorrhage during 7 years of clinical follow-up.
Coiling of aneurysms was performed on a biplane angiographic unit (Integris BN 3000; Philips Medical Systems, Best, the Netherlands) with the patient under general anesthesia. Before coiling or after placement of the first coil, a bolus of 2500 U heparin was administered intra-arterially followed by drip infusion of 1000 U of heparin per 500-mL infusion fluid during the intervention. Heparin was continued intravenously or subcutaneously for 48 hours after the procedure, followed by 80-mg aspirin daily for 3 months orally. For the 1 procedure with stent placement, the patient was preloaded with antiplatelet medication (clopidogrel 75 mg and aspirin 80 mg daily). Coiling was performed with Guglielmi detachable coils (GDC; Boston Scientific, Fremont, Calif) or Trufill DCS/Orbit coils (Cordis, Miami Lakes, Fla). The aim of coiling was to obtain an attenuated packing of the aneurysm, until not 1 coil could be placed.
Procedural complications (aneurysm rupture or thromboembolism) of coiling leading to death or neurologic disability at the time of hospital discharge were prospectively recorded in our data base during the weekly joint meeting. For comatose patients, thromboembolic complications were considered to have caused neurologic deficit if this was either clinically evident or if there were infarctions on subsequent CT scans in the territory of the involved vessel. Procedural rupture in comatose patients who subsequently died in the hospital was considered as procedural mortality.
Initial angiographic results of coiling were classified as (near) complete occlusion (90%–100%), incomplete occlusion (<90%), and not occluded. Aneurysm occlusion was determined in consensus during the weekly meeting.
Clinical and Imaging Follow-Up
Patients who survived the hospital admission period were scheduled for clinical follow-up in the outpatient clinic at 6 weeks and for angiographic follow-up at 6 and 18 months. In some patients treated with coil occlusion of the aneurysm including the PICA origin and/or vertebral artery occlusion, only MR imaging and MR angiography (MRA) follow-up was performed. Results of angiographic follow-up were classified in the same way as the initial postembolization occlusion. Incomplete occlusion at any point in time was considered an indication for further therapy, unless clinical or anatomic factors dictated otherwise. Clinical follow-up was assessed according to the Glasgow Outcome Scale (GOS) at every outpatient clinic visit and at every admission for follow-up angiography. Results and consequences of clinical and angiographic follow-up were discussed in the weekly meeting. When appropriate during the meeting, a decision was made concerning the need for additional treatment or extended angiographic or MRA follow-up. When additional treatment was performed, the result was evaluated in the weekly meeting and angiographic follow-up was scheduled at 6 months.
Results
Type of Treatments
Thirty-seven aneurysms in 36 patients were treated with selective occlusion of the aneurysm, sparing the parent PICA origin in 1 patient after stent placement (Enterprise; Cordis). Ten aneurysms with origin of the PICA from the aneurysm sac or with a wide neck based on the PICA were treated with different endovascular techniques: coil occlusion of the aneurysm including the parent PICA (internal coil trapping) in 2, coil occlusion of the aneurysm including the VA and PICA origin in 4, and coil or balloon occlusion of the VA proximal to the PICA in 4.
Initial Angiographic Occlusion and Complications
Initial angiographic occlusion of 47 PICA aneurysms was (near) complete in 39 (83%) and incomplete in 4 aneurysms (8.5%), and 4 aneurysms (8.5%) treated with proximal vertebral occlusion were not occluded. One patient treated with deliberate PICA origin occlusion developed lateral medullary and cerebellar infarctions leading to hemiparesis (Fig 6). Procedural rupture occurred in 9 aneurysms (19%), leading to death in 2 patients and to permanent disability in 1 patient. All procedural ruptures occurred in previously ruptured small (2–10 mm) aneurysms. Procedural ruptures were evenly distributed in the study period and were mainly caused by the microcatheter. Procedural mortality was 4.3% (2 of 46), and morbidity was 4.3% (2 of 46).
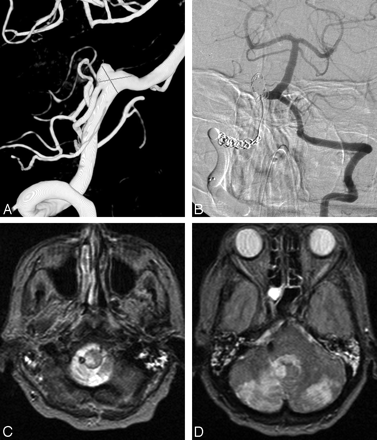
A 71-year-old woman presenting with acute SAH in poor clinical condition. A, 3D right vertebral angiogram shows broad-based PICA aneurysm with PICA originating from the sac. B, Right vertebral angiogram after coil occlusion of the aneurysm and coil and balloon occlusion of the right VA. C and D, MR imaging 2 weeks later shows right lateral medullary infarct and bilateral PICA infarctions. The patient was right hemiparetic.
Clinical Follow-Up and Rebleeding Rate
Clinical follow-up was available for all 46 patients. Six patients died during hospital admission: 2 after procedural complications and 4 of direct impact of SAH or diffuse vasospasm. One patient died of pneumonia 2 months after coiling in a nursing home. Clinical follow-up of 39 surviving patients was at a mean of 33.5 months (median, 24 months; range, 2–93 months; 109 patient-years). An 83-year-old patient died 3 months after coiling of cardiac disease in a nursing home. GOS score at 6 month follow-up for the remaining 38 patients was GOS 5 in 34 patients (89%) and GOS 3 in 4 patients (11%). During extended follow-up, 1 patient died of cardiac infarction 28 months after coiling. There were no episodes of recurrent or primary hemorrhage during 109 patient-years of follow-up. The annual incidence rate for recurrent or primary hemorrhage was 0% (95% confidence interval, 0%–4.1%).
Of 4 patients who presented with cranial nerve palsies, 1 was treated with selective coil occlusion of the aneurysm and the other 3, with proximal vertebral artery occlusion. Clinical symptoms resolved completely in all 4 patients.
Imaging Follow-Up
Of 40 patients (with 41 aneurysms) who survived the hospital admission period, 1 died before the 6-month follow-up interval. One patient refused follow-up angiography, and 1 patient is scheduled for 6-month follow-up. Of 38 eligible patients, 29 (with 30 aneurysms) had angiographic follow-up and 9 had MR imaging or CT follow-up. Mean duration of angiographic follow-up in 29 patients was 21.5 months (median, 16 months; range, 6–84 months). Three aneurysms reopened with time and were additionally coiled. Another 4 aneurysms were incompletely occluded (60%–80%), but additional coiling was not performed in view of unfavorable geometry of the aneurysm remnants. In one 74-year-old patient treated with proximal vertebral artery occlusion, the aneurysm remained open at 7-month follow-up angiography.
Nine patients had CT or MR imaging follow-up only. Three of these patients had been treated with proximal vertebral occlusion; in 2 patients who presented with cranial nerve palsies, this resolved in both and MR imaging findings suggested thrombosis of the aneurysmal lumen. The other patient had been treated after SAH. Follow-up MR imaging was equivocal for occlusion of the aneurysm, but there was no recurrent hemorrhage during 7 years of follow-up.
Six patients with CT or MR imaging follow-up only had been treated with coil occlusion of the aneurysm including the PICA origin with or without VA occlusion. In 3 of 6, small asymptomatic infarctions were evident in the territory of the occluded PICA.
Altogether, of 40 aneurysms with follow-up, 33 were (near) completely occluded, 5 aneurysms were incompletely occluded, 1 aneurysm was not occluded, and occlusion of 1 aneurysm was unknown.
Discussion
In this study, we found that endovascular treatment of PICA aneurysms is associated with technical problems in a substantial proportion of patients. Geometry of some PICA aneurysms precludes selective occlusion of the aneurysm with preservation of flow to the PICA. General techniques to treat wide-necked aneurysms such as balloon or stent-assisted coiling are often impossible in PICA aneurysms; deconstructive techniques with PICA occlusion or vertebral occlusion are the only endovascular options in these aneurysms. In this series, deliberate PICA-origin occlusion led to morbidity in 1 of 6 cases and to small asymptomatic cerebellar infarctions in another 3. In our opinion, occlusion of the aneurysm, including the PICA origin in patients presenting with acute SAH who are not good surgical candidates, is a valid option. In other cases, surgery, with or without bypass grafting8 might be a better alternative. Proximal VA occlusion, with the aim to reverse and decrease the flow to the aneurysm, was effective for alleviating symptoms of mass effect but did not lead to aneurysm occlusion by thrombosis in 2 of 4 patients. This type of therapy should be restricted to aneurysms presenting with mass effect that cannot be treated otherwise.
A remarkably high rate of procedural rupture occurred in PICA aneurysms treated with coil occlusion (21%, 9 of 43 treated with coil occlusion). A possible explanation, besides small aneurysm size, might be the geometry of the aneurysms and the VA: Virtually all PICA aneurysms project upward and slack in the microcatheter caused by the V3 loop of the VA can easily be transferred in an upward jump of the catheter during pushing, resulting in rupture of the aneurysm dome.
Only a few studies concerning endovascular treatment of proximal PICA aneurysms have been published so far. Clinical and angiographic results were reported to be favorable.9–12 In this study, procedural mortality was 4.6% (2 of 46, patients 25 and 29). Both patients were in very poor clinical condition after acute SAH and died several days and 2 months respectively after coiling complicated by rupture. Because our definition of procedural mortality is strict (comatose patients with procedural rupture who subsequently die in hospital), we assigned both patients as procedural mortality.
Surgery of PICA aneurysms is challenging because they are deeply located in front of the brain stem and surrounded by the lower cranial nerves. Postoperative temporary or permanent lower cranial nerve palsies occur in almost half of the cases6 with prolonged need for intensive care and inherent risk of developing pneumonia. With time, most of the lower cranial nerve palsies resolve. Despite these problems, overall results of surgery of PICA aneurysms are good.1–8 In experienced hands, PICA revascularization procedures can be performed in selected cases with good results.8
In the treatment of PICA aneurysms, a multimodality approach tailored to the specific aneurysm in the specific patient seems even more appropriate than for aneurysms at other locations. In patients presenting with acute SAH with an aneurysm suitable for coiling, coil occlusion of the aneurysm is the preferred treatment. When the PICA originates from the sac, coil occlusion of the aneurysm including the PICA origin should be considered because outcome is usually good. When patients present in a delayed time course after SAH or with unruptured aneurysms, surgical approaches may be a better alternative in selected cases. In aneurysms presenting with mass effect, endovascular proximal VA occlusion may be sufficient to alleviate symptoms.
Conclusions
In our experience, PICA aneurysms were challenging lesions, prone to procedural rupture. In some instances, endovascular treatment required occlusion of the parent PICA; usually this was well tolerated. In other instances, treatment required occlusion of the VA. Although this was effective in alleviating symptoms of mass effect, it was not effective in causing thrombosis of the aneurysm.
Footnotes
indicates article with supplemental on-line table.
References
- Received March 27, 2007.
- Accepted after revision April 30, 2007.
- Copyright © American Society of Neuroradiology