Abstract
BACKGROUND AND PURPOSE: Pineocytomas have been described as well-circumscribed, homogeneously enhancing masses. However, there is considerable variability in this appearance, and certain pineocytomas may have a predominantly cystic appearance on imaging. This has led some to suggest that differentiation between pineocytomas and pineal cysts may not be possible. We have attempted to determine if cystic pineocytomas could be found in a series of these tumors evaluated by CT and MR imaging.
MATERIALS AND METHODS: We searched the radiology literature as well as the medical records from our own institution for pathologically proved pineocytomas with available preoperative imaging or imaging reports, with specific focus on whether postcontrast MR imaging was included. In cases in which images were available, they were evaluated by a Certificate of Added Qualification (CAQ)-certified neuroradiologist and a radiology resident, who attempted to determine if the pineocytomas had any MR imaging characteristics of typical pineal cysts. To be considered a typical pineal cyst, an area of signal-intensity abnormality must be centered on the pineal recess, demonstrating internal homogeneity on T2-weighted imaging, following CSF signal intensity on T1- and T2-weighted images, without any marginal lobularity or nodular contrast enhancement and a wall thickness of <2 mm. In cases in which imaging was not available, radiology reports and/or descriptions provided in the radiology literature were reviewed by a CAQ-certified neuroradiologist. For any lesion described as cystic, we again attempted to elucidate the exact extent of imaging that was performed, note specific lesion characteristics, and determine if the lesion met the criteria described previously. Finally, for tumors in which image size was provided, the mean value of maximal tumor dimension, SD, median, and range were calculated.
RESULTS: Forty-four pathologically proved cases of pineocytomas from the radiology literature, as well as 8 pathologically proved cases of pineocytomas from our institution with available imaging studies and/or reports, were reviewed. Of these, 23 were solid masses, and 7 were partially solid and cystic, whereas 14 tumors could not be completely characterized due to incomplete imaging evaluation. Eight were primarily cystic; however, none of these could be confidently characterized as meeting the criteria for a typical cyst.
CONCLUSION: In our analysis, no truly cystic pineocytomas were identified.
Pineocytomas are rare tumors that arise from pineal parenchymal cells. Imaging features are nonspecific; classically, they present as well-circumscribed homogeneously enhancing solid masses, centered on the pineal gland. However, there is considerable variability in this appearance, and certain pineocytomas may have a cystic or partially cystic appearance. In some cases, the appearance may be similar to a pineal cyst, and differentiation between these entities may not be possible.1–13
Pineal cysts are benign cystic lesions identified in 1%–4% of MR images of healthy subjects.14 Although some authors advocate only clinical follow-up for typical asymptomatic pineal cysts, this is not yet widely accepted, and it has been reported that a pineocytoma can mimic a typical pineal cyst in imaging appearance. Therefore, these lesions are often followed up with multiple imaging studies to document stability, and, at times, surgical intervention is even considered to obtain a definitive diagnosis.1–16
It has been reported that on delayed imaging (60–90 minutes after gadolinium injection), contrast material may diffuse from the enhanced rim into the fluid center of the pineal cyst, producing an increasingly homogeneous enhancement pattern, suggestive of solid neoplasm.12,14,17 Therefore, it is important to take into account the time elapsed after contrast administration in such unusual cases.
We attempted to determine if the truly cystic pineocytoma exists when imaged with multisequence MR imaging with contrast or if it is a myth, which needlessly concerns radiologists and causes the recommendation of possibly unnecessary follow-up studies and potential surgical intervention, placing the patient at increased and unnecessary risk.
Materials and Methods
Literature Review
A PubMed search was performed on May 23–24, 2006, and subsequently repeated on April 24–25, 2007, to ensure that no cases were missed, by using the keywords “pineocytoma,” “pineal cyst,” “pineal tumor,” “pineal neoplasm,” “pineocytoma MR,” “pineocytoma imaging,” “pineal cyst imaging,” and “pineal MR.” The literature was searched for case reports and case series from the past 20 years in English-language articles describing the appearance of pineocytomas on MR imaging.
Internal Patients
Our institution has in place an “honest broker” system to comply with the Health Insurance Portability and Accountability Act regulations, and this study was performed within the guidelines of that system. A search was performed of our composite electronic medical records, encompassing 20 academic and community hospitals affiliated with our institution in an effort to identify patients who had pathologically proved pineocytomas with available imaging studies and/or reports. Radiology reports from January 1, 1991, to October 4, 2006, were searched by using the keywords “pinealcytoma,” “pineocytoma,” “pinealblastoma,” “pineal blastoma,” “pineoblastoma,” “pineal parenchymal,” and “pineal tumor.” Pathology reports from January 1, 1991, to October 4, 2006, were searched by using keywords “pineocytoma” and “pineal parenchymal tumor” to attempt to uncover any additional cases. A total of 322 cases were reviewed, excluding duplicated names between the 2 searches. Final cases were selected that met the following 3 criteria: A CT and/or MR imaging was performed before surgery, the images and/or radiology reports were available, and there was an available pathology report confirming the diagnosis of pineocytoma.
For both the cases from the literature and the internal patients, when images were available, they were reviewed by a Certificate of Added Qualification–certified neuroradiologist and a third-year radiology resident, and the imaging features of these lesions were evaluated with respect to the presence of cysts, enhancement pattern, lesion size, and wall characteristics. Note was made of any limitations of the available images. When only reports were available, we attempted to obtain relevant information regarding these features. If available, lesion size was noted or measured, and average maximal tumor diameter with SD, median, and range was calculated. Where possible, we noted as many as possible of the following technical scanning criteria: section thickness, sequences used (for MR imaging), imaging plane, use of contrast, dose, matrix size, FOV, any delay between injection and imaging, scanner make and model, and field strength (for MR imaging).
Finally, we attempted to classify tumors as solid or cystic, keeping in mind our goal of attempting to determine whether a truly cystic pineocytoma existed and if it could possibly mimic a typical pineal cyst on imaging. For the purposes of this study, the criteria of a typical pineal cyst put forth by Barboriak et al were used.7 A typical pineal cyst was considered present if it met 4 MR imaging criteria: a round or ovoid area of signal-intensity abnormality centered on the pineal recess, the area of concern demonstrating hypointensity to white matter on T1-weighted images and isointensity with CSF on T2-weighted images, the area of concern being internally homogenous on T2-weighted images, and last, no marginal lobularity or nodular contrast enhancement demonstrated. A rim of T2-hypointensity or contrast enhancement on T1-weighted images was permitted if the rim measured <2 mm thick.7
Results
Literature Review
Twelve case reports or case series that included MR imaging of pineocytomas, some of which also included CT imaging, were found and yielded 44 pathologically proved cases of pineocytomas. Various imaging techniques, including CT with and without contrast and T1-weighted, T2-weighted, and postcontrast T1-weighted MR imaging, were performed. Thirteen of 44 cases were imaged with non-contrast-enhanced CT. Sixteen of 44 cases were imaged with contrast-enhanced CT, and 37 of 44 were imaged with MR imaging. Of those, 34 of 44 were imaged with T2-weighted MR pulse sequences, 36 of 44 were imaged with T1-weighted MR pulse sequences, and 21 of 44 cases were imaged with T1-weighted MR pulse sequences following the administration of gadolinium contrast.
Unfortunately, 5 of the 12 reviewed case reports or case series provided no specific information regarding scanner type used or scanning parameters. The remaining 7 studies provided differing levels of detail regarding scanning parameters. MR imaging was performed on 0.5T, 1.5T, and 2T scanners. T2-weighted images were acquired with a conventional spin-echo technique with TRs ranging from 2000 to 4800 ms and TEs ranging from 80 to 108 ms. Section thickness (provided in only 2 studies) ranged from 3 to 7 mm. For T1-weighted spin-echo sequences, TRs ranged from 400 to 600 ms and TEs ranged from 11 to 30 ms. Section thickness ranged from 3 to 7 mm. Information was provided in only 1 study regarding FOV, 22 × 22 cm for both T1 and T2 sequences, and matrix size, 256 × 256 for T2 sequences and 256 × 296 for T1 sequences. No information was provided regarding the specific type of gadolinium-based contrast used, volume of contrast, or time elapsed between contrast administration and imaging.5,14,18–22
Only a single reviewed study provided information regarding CT scanning parameters, though even then specific information on scanner type was not provided. In that study, 10-mm sections were obtained in the supratentorial region, and 5-mm sections, in the infratentorial region, using 200–275 mAs and 100–120 KV(p), with a 512 × 512 matrix. Information was not provided in any study regarding type or volume of iodinated contrast used or time from contrast administration to imaging.18
Of the tumors evaluated, 16 were solid masses, whereas 6 were partially solid and partially cystic. These tumors were well-circumscribed isoattenuated-to-hypoattenuated masses, centered on the pineal region on precontrast CT, with marked enhancement on postcontrast CT. On MR imaging, they were well-circumscribed masses, demonstrating low-to-isointense signal intensity on T1-weighted imaging, and heterogeneous, isointense-to-high signal intensity on T2-weighted imaging. Eleven of the aforementioned 22 solid or partially solid masses were imaged by using postcontrast T1-weighted imaging, all of which demonstrated enhancement of the solid portion of the mass. Unfortunately, 14 tumors could not be precisely classified because they were incompletely evaluated, lacking either T1-weighted, T2-weighted, or postcontrast T1-weighted MR imaging.
Two cases series described primarily cystic pineocytomas. The first described 6 pineocytomas that were primarily cystic.5 One was septate and eccentric with a thick wall, 1 had a dorsal nodule, 3 had walls that were thicker than 2 mm (more precise measurements were not provided), whereas 1 may have been a simple cyst with a thin wall (it is difficult to determine this from the published data). Unfortunately, specific information regarding wall thickness, specifically whether the enhancing wall was greater or less than 2 mm, was not provided, nor were images provided for independent review. The second described 2 cystic pineocytomas, both of which were symptomatic—headaches and tics in 1 case and headache, vertigo, Parinaud syndrome, and balance disturbances in the second—and both of which demonstrated enhancement of the cyst wall. Unfortunately, again further information regarding wall nodularity or wall thickness, specifically whether the enhancing wall was greater than or less than 2 mm, was not provided; images were not provided for independent review, nor were scan parameters available.23
Very few reviewed cases provided detailed measurements of tumor size. Only 8/44 cases provided tumor measurements in 2 or 3 dimensions, whereas 2/44 cases provided only the maximal tumor dimension.6,19,22 The remaining cases either classified the tumors as “small,” “medium,” “large,” or “>20 mm,” or did not comment on tumor size.8,19–21,23–27 Given these limitations, we calculated the average maximal dimension of the 10 tumors with provided measurements to be 22.1 mm with an SD of 13.2 mm. Values ranged from 9 to 50 mm, with a median value of 22.5 mm.
Internal Patients
The records of our institution yielded 8 pathologically proved cases of pineocytomas with available MR imaging studies and/or reports. Various imaging techniques, including CT without contrast and T1-weighted, T2-weighted, and postcontrast T1-weighted MR imaging, were performed. Three of 8 cases were imaged with non-contrast-enhanced CT, 4/8 were imaged with T2-weighted MR pulse sequences, 4/8 were imaged with T1-weighted MR pulse sequences, and 8/8 cases were imaged with T1-weighted MR pulse sequences following the administration of gadolinium (though in 2 cases tumor enhancement was not specifically mentioned in the report and images were not available for independent review).
CT was performed on conventional third- and fourth-generation CT scanners (GE Healthcare, Milwaukee, Wis). Five-millimeter sections were obtained at 280–300 mAs and 120–140 kV(p), using a 512 × 512 matrix. MR imaging was performed on a 1.5T magnet (GE Healthcare). T2-weighted images were acquired with a conventional spin-echo technique with TRs ranging from 3000 to 3500 ms and TEs ranging from 98 to 128 ms. Section thickness was 5 mm with a 1-mm intersection gap. For T1-weighted spin-echo images, TRs ranged from 400 to 700 ms and TEs ranged from 9 to 14 ms. Section thickness ranged from 3 to 5 mm with an intersection gap ranging from 0.5 to 1.0 mm. Postcontrast imaging was performed by using Optimark or Multihance gadolinium-based contrast material (Tyco Healthcare/Mallinckrodt, St. Louis, Mo; Bracco, Milan, Italy), and the volume of contrast used ranged from 20 to 25 mL. At our institution, postcontrast imaging of the brain is performed immediately (<1 minute) after contrast administration. All patients had images obtained in at least 2 orthogonal directions. FOV used ranged from 200 to 220 mm, depending on patient size; matrix size was 256 × 192 for T1 pulse sequences and 320 × 224 for T2 pulse sequences. Maximal pixel size in the plane of imaging, therefore, ranged from 1.28 × 0.96 mm to 1.60 × 1.12 mm.
Seven tumors were solid masses, whereas 1 was partially solid and partially cystic.
Four tumors were incompletely evaluated, lacking either T2-weighted MR imaging or precontrast T1-weighted imaging (stereotactic localization studies done at our institution use only T1 postcontrast imaging). On postcontrast T1-weighted imaging, all tumors demonstrated enhancement of the solid portion of the mass. Notably, no tumor had the appearance of a thin-walled simple cyst on postcontrast imaging.
Of the reviewed tumors, 6 of 8 either had radiology reports that provided tumor dimensions in 2 or 3 planes or such data were obtainable on image review; 1 of 8 cases in which only the report was available provided only a single maximum tumor dimension, whereas a single image report made no comment on tumor size. Average maximal tumor dimension was calculated to be 18.9 mm with an SD of 5.5 mm; values ranged from 13 to 27 mm, with a median value of 18 mm.
Discussion
Determination of the natural history and exact imaging appearance of pineocytomas is difficult due to the rarity of this disease. It has been previously reported that pineocytomas can mimic typical pineal cysts in imaging appearance1–13; it has been our experience, however, that when fully and completely imaged—which necessitates using postcontrast MR imaging—most of these tumors bear little resemblance to typical pineal cysts.
A typical pineal cyst, as defined by Barboriak et al,7 can have a thin <2-mm rim of enhancement. It is the lack of a blood-brain barrier surrounding the pineal gland that allows the walls of these benign cysts to enhance because the wall is composed of pineal tissue.17 It has been theorized that the delayed internal enhancement of pineal cysts is most likely due to passive diffusion of contrast material from surrounding pineal tissue with a possible component of active secretion, as well; however, the exact mechanism has yet to be fully described.17 This same mechanism is responsible for the increasingly homogeneous pattern of enhancement of pineal cysts, possibly resembling a solid mass, on significantly (60–90 minutes) delayed postcontrast imaging.17 This is a potential pitfall and may result in confusion when interpreting delayed images (which are not routinely or intentionally obtained at most imaging centers). Therefore, it is important to take into account the time elapsed after contrast administration in such unusual cases.
The imaging features of atypical pineal cysts, including irregular nodular enhancement and hemorrhage into a typical pineal cyst, have been previously described by multiple authors.7,9,14,15,17 Large pineal cysts may cause mass effect and compression on the quadrigeminal plate or vein of Galen, leading to lethal increases in intracranial pressure and neurologic devastation.17 Some authors advocate imaging follow-up for pineal cysts >14 mm for this reason, whereas other authors report that pineal cysts are unlikely to change in size with time and may even involute, and they advocate follow-up on a clinical basis alone, with future imaging based on patient symptom.7,16,17 Clearly any patient presenting with symptoms referable to a pineal cyst (or other pineal mass) deserves imaging follow-up. To our knowledge, however, no study has specifically correlated cyst size with malignant potential.
Currently, some authors advocate imaging follow-up for typical pineal cysts to document stability with time, particularly in cysts larger than 10–14 mm, under the assumption that they may further enlarge or become symptomatic, whereas others advocate only clinical follow-up if there are no atypical features or symptoms related to the pineal cyst.7,9,15,16 Some radiologists also recommend follow-up under the assumption that if the cyst changes in size or develops atypical features with time, then it could represent a neoplasm such as a pineocytoma, though this is a specific recommendation made in only 1 of the series we reviewed, which recommended follow-up “for many years” for all pineal cysts.23
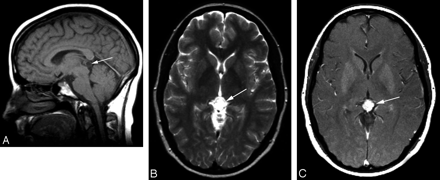
The importance of postcontrast T1-weighted MR imaging in evaluating these tumors cannot be overstated because pineocytomas may have an imaging appearance identical to that of pineal cysts on noncontrast CT, T1-weighted precontrast MR imaging (Fig 1A), and T2-weighted precontrast MR imaging (Fig 1B). However, on immediate postcontrast imaging, pineocytomas will generally show either internal or nodular wall enhancement (Fig 1C), as opposed to typical pineal cysts, which will not significantly enhance or will only have enhancement of a thin wall on immediate postcontrast imaging.
Two case series did describe 3 pineocytomas that may have been indistinguishable from pineal cysts on imaging, including postcontrast MR imaging.5,23 All 3 of the tumors in question were noted to demonstrate cyst wall enhancement on postgadolinium imaging; however, specific characteristics of the cyst wall, namely if it was greater or less than 2 mm in diameter, were not mentioned. Unfortunately, the actual images were not provided for independent review in any of these cases. This finding appears to represent an unusual manifestation of a rare tumor because no additional such examples could be found in the literature or in our institutional records. It is unfortunate that these noteworthy cases are not more thoroughly presented because they are the only 3 cases in the literature describing an appearance of a pineocytoma identical to that of a simple cyst, so verifying that they truly met the criteria for simple cysts would be critical in the determination of whether pineocytomas can actually ever have this appearance. In 2 of the 3 cases, the pineocytoma caused clinical symptoms, which resulted in the patient's seeking medical attention.
On the basis of our findings, we advocate that if a lesion meets the aforementioned criteria for a typical pineal cyst as set forth by Barboriak et al7 (<2 mm thick, no nodularity, etc), then no imaging follow-up is necessary to exclude a pineocytoma. There have been reports of pineal cysts enlarging with time, although this is rare and cysts may even involute; if a patient with a known pineal cyst develops new symptoms, then further imaging would be warranted. For a lesion that does not meet the criteria for a typical pineal cyst on MR imaging or causes clinical symptoms, further imaging and possibly even tissue sampling may be warranted. Although no specific recommendations regarding follow-up of cysts discovered incidentally on CT scans was garnered from our review of the literature, we advocate that if a lesion is 1 cm in minimal diameter or greater, then it is worthwhile confirming that the lesion has features of a simple cyst on MR imaging. Again, the importance of postcontrast thin-section MR imaging in this situation cannot be overstated because some pineocytomas may be indistinguishable from pineal cysts on CT and precontrast T1- and T2-weighted MR imaging; however, in the overwhelming majority of cases, they will show nodular wall or internal enhancement on postcontrast imaging. Although there is no clear lower limit in size for pineocytomas, most appear to be 1 cm or larger, and it would be impractical to perform MR imaging on every incidentally discovered pineal cyst. Therefore, 1 cm seems like a reasonable threshold.
The principal limitation of our study was the number of pineocytomas sampled. This is a reflection of the rarity of this disease and the infrequent occurrence of these tumors, even in large tertiary care centers. Further research in this area with a multicenter trial, pooling the resources of several large institutions in an attempt to obtain a more homogeneous group of pathologically proved pineocytomas with available CT and MR imaging to review, would be worthwhile. An associated secondary limitation is the relatively poor description of the imaging appearance of pineocytomas and pineal cysts available in the literature reviewed, along with a lack of provided images in most cases (including potentially purely cystic ones), which makes subtle differentiation between these 2 entities challenging. Also, the overall heterogeneity and lack of details of imaging techniques used add additional confounding factors, such as accurately determining wall thickness or the presence of subtle wall nodularity.
The data from our own institution are heterogeneous because many of our own patients with pineocytomas have received various types of imaging using different techniques; some patients had images available, while others did not. To some extent, this can be attributed to a transition within our institution from printed film to PACS, a change in scanners and protocols, the fact that some patients had full diagnostic imaging at outside institutions and were imaged at our institution only for stereotactic localization, and the fact that some of the older images were destroyed and not archived. Nevertheless, a more uniform imaging approach would certainly have been helpful because these factors limited our ability to fully evaluate the lesions, particularly for precise measurements of a cyst wall, though this was not an issue in these patients because none of these lesions were entirely cystic with a thin wall on postcontrast imaging and, therefore, did not require measurements for differentiation from a simple cyst.
Conclusion
In summary, although 2 case series described a single asymptomatic pineocytoma and 2 clinically symptomatic pineocytomas that were reportedly indistinguishable from typical pineal cysts on imaging, these cases were incompletely documented, and this seems to be an unusual occurrence of a rare tumor, on the basis of our experience and the remainder of reports in the literature. Most of these tumors will be at least partially enhancing masses that may contain areas of cystic change or at least nodularity of their walls, which should not be confused with typical pineal cysts. Furthermore, typical pineal cysts should not require imaging follow-up, unless the patient develops new symptoms.
{/PICK;0750f1;;;page;;;;yes;1} {/GRAPH;;comptd;;center;stack} {/CAPT;;;center;stack;4224n}
A, Pineocytoma on precontrast MR image. Sagittal T1-weighted MR image (TR/TE = 500/17 ms) shows an ovoid lesion (arrow) that is hypointense to white matter and close to CSF in signal intensity, with a thin intermediate signal-intensity rim centered on the pineal recess. B, Pineocytoma on precontrast MR image. Axial T2-weighted MR image (TR/TE = 4000/90 ms) shows a homogeneous ovoid lesion (arrow), which is isointense to CSF in signal intensity with a thin intermediate-intensity rim centered on the pineal region. C, Pineocytoma on postcontrast MR image. Axial postcontrast T1-weighted MR image (TR/TE = 760/17 ms) shows a homogeneously enhancing mass (arrow) centered on the pineal recess. The homogeneous solid enhancement excludes the possibility of this tumor being cystic.
Footnotes
Paper previously presented at: Annual Meeting of the American Society of Neuroradiology, June 9–14, 2007; Chicago, Ill.
References
- Received March 22, 2007.
- Accepted after revision May 22, 2007.
- Copyright © American Society of Neuroradiology