Abstract
BACKGROUND AND PURPOSE: Rhabdoid meningioma (RM) is a recently described variant of malignant meningioma, with radiologic features currently not well characterized in the medical literature. The purpose of this study was to describe and characterize clinical features and imaging findings associated with RM.
MATERIALS AND METHODS: CT (n = 8) and MR (n = 15) images of 15 patients (4 men and 11 women; mean age, 52 years; range, 22–75 years) with 16 pathologically proved RMs along with associated clinical records were retrospectively reviewed. All of the patients underwent surgical resection and had additional radiation therapy except for 1 patient. After surgery, the patients had follow-up brain MR imaging to evaluate for tumor recurrence.
RESULTS: Nine lesions (56%) were located in the cerebral convexity, and 4 lesions (25%) were located in the parasagittal areas. The tumors were isointense (n = 15) to gray matter on T1-weighted images, whereas they were hyperintense (n = 14) on T2-weighted images. On gadolinium-enhanced T1-weighted images, homogeneous enhancement was seen in 10 lesions, and heterogeneous enhancement was seen in 6 lesions that had cysts. Cystic components were noted in 6 lesions (38%). Severe peritumoral edema was seen in 12 lesions (75%). Nine lesions (56%) had hyperostosis, and 5 of them also had bone destruction. Among the 8 cases with initial CT scans, only 1 had amorphous calcifications (13%). There was only 1 recurrence of RM found during the follow-up period after surgical resection.
CONCLUSION: RMs tend to have prominent peritumoral edema, cystic components, and bone involvement.
Meningiomas are the most common nonglial primary brain tumor and account for 15%–20% of all primary brain tumors. It is the most common intracranial extra-axial neoplasm.1 They have a wide range of histopathologic appearances; the World Health Organization (WHO) 2000 classified some subtypes displaying aggressive characteristics, such as atypical, clear cell, and chordoid meningiomas, as WHO grade II (atypical meningioma) and papillary and anaplastic meningiomas as WHO grade III (anaplastic or malignant meningioma).2 Atypical and anaplastic meningiomas have more aggressive behavior with more recurrences. Typical meningiomas have recurrence rates of approximately 7%–20% and atypical meningiomas have rates of 29%–40%, whereas anaplastic meningiomas tend to reappear in 50%–78% of cases.3–5
Rhabdoid meningioma (RM) was first described in 1998 as an unusual variant of meningiomas.6 It has an increased proliferative activity and is classified as a WHO grade III meningioma.6,7 According to the literature, most RMs behave aggressively and have a very poor prognosis.6–8 It is important to recognize rhabdoid morphology in a meningioma early to help in both the diagnosis and understanding of its clinical course.
Although the clinical and pathologic findings of RM have recently been well known,6,8–10 the radiologic features of RM have been rarely addressed in the literature. The goal of this study was to investigate CT and MR findings, as well as clinical features, present in 15 patients with 16 histologically proved RMs.
Materials and Methods
Patients
Between January 2001 and May 2006, we experienced a total of 15 patients with 16 pathologically proved RMs from our institution. The diagnosis of RM depends on finding evidence of meningoepithelial differentiation either by microscopic findings, such as whorls or intranuclear pseudoinclusions, or immunochemistry characteristics, such as expression of vimentin and or epithelial membrane antigens. The patients in the study included 4 men and 11 women, with ages ranging from 22 to 75 years (mean age, 52 years). A retrospective analysis using medical records, CT, and MR findings was done to evaluate the clinical and radiologic features of RM.
The initial symptoms of the patients and subsequent treatments, such as surgery and radiation therapy, along with the follow-up clinical status after the surgery, were evaluated based on the patients’ medical record. All of the patients underwent surgical resection of the tumor and had additional radiation therapy except for 1 patient (not able to undergo radiation therapy because of poor physical condition). After surgery, they were followed every 3–6 months for the first year and then annually with brain MR imaging to evaluate for tumor recurrence.
Imaging
CT scanning was performed in 8 patients and MR imaging in all 15 patients. The CT scans were obtained with a spiral CT scanner (HiSpeed; GE Medical Systems, Milwaukee, Wis) with 120 kvp and 150 mA. The enhanced scans were obtained after the injection of 150 mL of iopromide (Ultravist 300; Schering, Seoul, Korea). All of the MR examinations were performed on a 1.5T MR unit (Signa Advantage Horizon; GE Medical Systems). Axial T1-weighted images (TR/TE/NEX, 400–550 ms/11–14 ms/2; FOV, 21 cm; section thickness, 5 mm with a 1 mm gap; matrix size, 256 × 256) and axial T2-weighted images (TR/TE/NEX, 2500 ms/80 ms/1; FOV, 21 cm; section thickness, 5 mm with a 1 mm gap; matrix size, 256 × 256) were obtained. In addition, contrast-enhanced T1-weighted images were obtained in the axial, sagittal, and coronal planes after the intravenous injection of 0.1 mmol/kg of gadolinium dimeglumine.
Two neuroradiologists retrospectively reviewed all of the CT and MR images for consensus. We investigated the CT and MR imaging characteristics, with particular attention on the location and size of the mass, presence of necrosis or calcification in the tumor, bone change such as hyperostosis or bony destruction, peritumoral edema, and intratumoral or peritumoral cystic change. The signal intensity of the tumor and the pattern of enhancement were also evaluated. The signal intensity of the tumors was compared with gray mater. The locations of the tumor were also analyzed: parasagittal, convexities, sphenoid ridge, olfactory groove, parasellar, and posterior fossa. Peritumoral edema was graded as absent, mild (extending <1 cm from the outer margin of the mass), moderate (1–4 cm with mild mass effect), or severe (>4 cm with marked mass effect including midline shift).11 The size of the tumor was obtained by measuring the greatest width of the enhanced tumor in all of the sections.
Results
Clinical Presentation
Most patients had nonspecific symptoms, including memory disturbances, dizziness, headache, seizure, and vomiting. Two patients with a mass in the sphenoid ridge had exophthalmoses. The average symptom duration was 5 months (ranged from 2 days to 6 months) except for 2 patients who had a 2-year history of headache. Surgical tumor removal was performed in all of the patients, and additional radiation therapy was provided except in 1 patient who was in very poor general condition. Radiation therapy was started approximately 3–6 months after operation, and the total radiation dose was 5400 rads. Five patients who had residual tumors were treated with additional gamma knife surgery.
The postoperative follow-up period for the patients ranged from 11 to 39 months (mean, 23.1 months). During the follow-up period, 3 of the 5 residual lesions increased in size. Two of them were confirmed as postradiation necrosis via biopsy, and the other was considered to be due to postradiation necrosis by the follow-up MR studies. There was 1 recurrent patient who had not undergone radiation therapy because of poor physical condition who had mass recurrence along the resection margin at 34 months of follow-up.
CT and MR Imaging Findings
A total of 16 masses were observed among the 15 patients studied. Nine (56%) of 16 lesions were located in the cerebral convexity, 4 (25%) were parasagittal, and 3 (19%) were located along the sphenoid ridge. The tumors ranged from 10 to 60 mm in diameter, and the mean diameter was 35 mm.
The tumors were either hypointense (n = 1) or isointense (n = 15) to gray matter on T1-weighted images and isointense (n = 2) or hyperintense (n = 14) on T2-weighted images (Fig 1). On gadolinium-enhanced T1-weighted images, homogeneous enhancement was seen in 10 lesions, and another 6 lesions that had cystic components displayed heterogeneous enhancement.
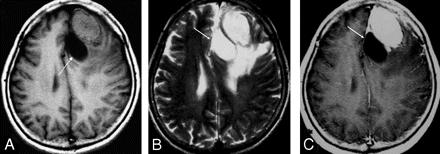
A 64-year-old woman presenting with memory disturbances. Axial T1-weighted (A), T2-weighted (B), and contrast-enhanced T1-weighted (C) MR images show a marked enhancing mass at left frontal lobe. Note peritumoral cyst (arrow) and extensive peritumoral edema surrounding the tumor.
Twelve lesions (75%) had severe peritumoral edema (>4 cm with marked mass effect, including midline structure shifting). Nine lesions (56%) had hyperostosis, and 5 lesions also had bony destruction (Fig 2). Cysts were seen in 6 lesions (38%): 5 peritumoral cysts and 1 intratumoral cyst. Among all of the patients who also underwent a CT scan (n = 8), only 1 had amorphous calcifications (13%) present.
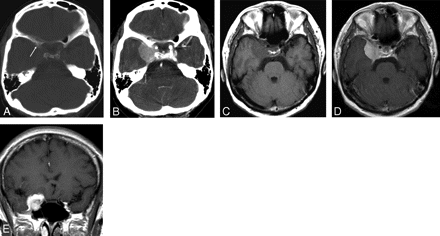
A 56-year-old woman presenting with right eyelid palsy. A, Axial precontrast CT image demonstrates hyperostosis and bony erosion (arrow) at the right anterior clinoid process. B, Postcontrast CT image shows a well-enhancing mass arising from right sphenoid ridge. C–E, Axial T1-weighted (C), contrast-enhanced axial (D), and coronal (E) T1-weighted MR images show a marked enhancing extra-axial mass at the sphenoid ridge.
Discussion
Clinical Presentation
RM occurs mainly between 40 and 60 years of age (mean age, 52 years) and had female predominance (female-to-male ratio, 11:4; 73% female) in our study. This is similar to other previously reported cases of RM in the literature where the mean age at presentation was 46.1 years (range, 9–84), also with a female predominance (female-to-male ratio, 24:16).8–10,12–15 Atypical and malignant meningioma usually present earlier than benign meningioma.4 However, in our study, the age population of the RM was not significantly different from benign meningiomas. The female predominance in meningiomas might be because of sex hormones, and progesterone may play an important role in the development, growth, and regression of meningiomas.16 Actually, progesterone receptor positivity has been reported to negatively correlate with tumor grade by many authors.16–19 In other words, there is a reduction of progesterone receptors from WHO grade I to grade II and a progesterone receptor negativity in WHO grade III tumors.16–19 The reason why RM presents similarly to benign meningioma is not clear. However, this may be because of the fact that the rhabdoid morphology can only present in a part of the tumor, though most RMs have high proliferative indices and additional histologic features of malignancy.2,8,20
The patients had variable presenting symptoms depending on the location of the tumors; seizure, hemiparesis, and gait disturbance for convexity or parasagittal locations; eyelid paralysis; and exophthalmoses in sphenoid lesion with orbit involvement. Most of the patients with RM had variable symptom durations (average, 5 months; range, from 2 days to 2 years).
Perry et al8 showed in their series of 15 RMs that 87% of patients had at least 1 recurrence, 13% had extracranial metastasis, and 47% died of disease at follow-up. Median time to death was 5.8 years after initial surgery and 3.1 years after the first appearance of rhabdoid morphology.8 In comparison, there was only 1 recurrence (7%) and no extracranial metastasis or death in our study. The reason for this low recurrence rate is not clear. It may be because of the radiation therapy, which was performed on a larger number of our case subjects compared with the study by Perry et al,8 and some of the discrepancy may be because of the relatively short follow-up from the current article. Fourteen (93%) of 15 patients in our study underwent both surgery and radiation therapy. The 1 case of recurrence occurred in the patient who was not able to undergo radiation therapy because of postoperative poor general condition. The role of adjuvant radiation therapy on meningioma remains unclear, but appears to be effective in the treatment of malignant meningiomas.21,22 However, the relatively short postoperative follow-up period (ranged from 11 months to 39 months; mean, 23.1 months) can also attribute some of the differences in recurrence rates. Further long-term follow-up studies will be needed.
Imaging Features
The cerebral convexities (56%) and the parasagittal region (25%) were common sites. There were no cases arising in the olfactory groove, parasellar region, and posterior fossa in our study. Nine (56%) of 16 tumors had a maximum diameter more than 40 mm (mean diameter, 35 mm; range, 10–60 mm).
RM showed isosignal intensity (15 of 16 tumors [94%]) to gray matter on T1-weighted images and high signal intensity (14 of 16 tumors [88%]) on T2-weighted images. On gadolinium-enhanced T1-weighted images, homogeneous enhancement was seen in most of the lesions (62%). Six RMs (38%) showed heterogeneous enhancement because of cystic components in the tumors. There are many studies suggesting correlations between MR signal intensity and subtypes of meningiomas.11,23 According to the previous study, syncytial or angioblastic meningiomas tend to show hyperintensity on T2-weighted images, and fibroblastic or transitional meningiomas tend to show hypointense signals on T2-weighted images.11,23 However, the relationship between signal intensity and histologic subtypes still remains controversial. With intravenous contrast administration, homogeneous attenuated enhancement is generally the rule with meningiomas. However, they may have a heterogeneous enhancement, and these features can be seen in both malignant and aggressive histologic variants or even in typical meningiomas that have cysts, hemorrhaging, or necrosis.11,23
Incidence of cystic component was relatively high in our RMs (38%) compared with typical meningiomas (4%–7%).24,25 Six of 16 RMs had cystic components: 5 peritumoral cysts and 1 intratumoral cyst. These 2 cyst types have quite different formation mechanisms. Intratumoral cysts are caused by biologic changes within the tumor, such as tumor necrosis or degeneration, whereas extratumoral ones appear to be secondary to local hydrodynamic changes and disturbance of CSF reabsorption.26,27 There are some reports about the relationship of malignant meningiomas and higher incidence rates of cyst formation.28,29 Vassilouthis and Ambrose29 included cystic component as one of the CT criteria to evaluate histologic aggressiveness of meningiomas.
Peritumoral edema was seen in 75% of our RM cases (12 of 16 lesions). All of them showed severe edema. Edema can often present in all types of meningiomas. There have been many reports suggesting no correlation between the degree of edema and the histologic types and size of the meningioma.23,30 Some authors, however, have reported increased edema in angioblastic and meningothelial meningiomas.11,23,27,30–32 The most important contributing factor of peritumoral edema is based on the attenuation of the vascular network present in the tumor.11,23
In our study, calcification was not a dominant feature in RM. Among the cases that had an initial CT scan (n = 8), only 1 had amorphous calcifications (13%) in the tumor. Calcification may be present in up to 15%–20% of cases of meningioma and can take different forms, including chunky, rimlike, punctuate, or diffuse. Some authors have pointed out that calcifications are absent or scant in malignant meningiomas.29
More than half of the lesions (n = 9; 56%) had bony hyperostosis, and more than half (n = 5) of those had additional bone destruction visualized. Bone changes occur in approximately 20%–25% of all types of meningiomas.33 Such changes may take the form of mild blistering or hyperostosis or they may be destructive. Bone involvement, especially osteolysis, has been mentioned as a factor in helping to predict the malignancy of meningioma.33
There were many trials to find out whether radiologic features can predict the subtype and prognosis of meningioma.11,23,29,33,34 Some imaging features are known to be associated with aggressiveness, such as bone destruction, central areas of necrosis, indistinct tumor margins at the brain surface, and irregular inward projections of tumor.34 However, the findings are far from specific and have limitations for prognosis prediction. These characteristics do not seem to be useful in distinguishing RMs from other high-grade meningiomas or even benign meningiomas with any degree of certainty.34
The limitations of this study included a relatively short follow-up period and a small number of cases for review. The imaging findings are not specific for RM, and these characteristics certainly do not seem to be useful in distinguishing RM from other high-grade meningiomas in daily practice. Further long-term follow-up studies and a comparative study between RM and high-grade meningiomas are needed.
In summary, RM more often tends to have cystic components, prominent peritumoral edema, and bone involvement based on our study. RM also appears to have a female predominance and not-so-poor prognosis as originally thought. Although the improved prognosis may be attributed to the added effects of adjuvant radiation therapy, further long-term follow-up studies are needed.
References
- Received November 28, 2006.
- Accepted after revision January 16, 2007.
- Copyright © American Society of Neuroradiology