Abstract
BACKGROUND AND PURPOSE: Delayed cerebral ischemia from vasospasm is a major complication after aneurysmal subarachnoid hemorrhage (SAH), but complications and/or low efficacy are associated with current therapy. We report our initial experience with intra-arterial use of a calcium channel blocker, nicardipine.
MATERIALS AND METHODS: A retrospective review of a consecutive series of patients with clinical and angiographic vasospasm treated with intra-arterial nicardipine was performed. Standard criteria for definition of significant, intractable vasospasm after aneurysmal SAH were used. After catheter angiographic confirmation of vasospasm, arteries showing severe narrowing were targeted for superselective catheterization. Nicardipine was infused at a high dose rate (0.415–0.81 mg/min). Contrast injections were performed at 2–5-mg intervals to assess effective response (a 60% increase in arterial diameter of the most severely decreased in caliber vessel compared with the very first angiographic run).
RESULTS: Eleven consecutive patients underwent a total of 20 procedures; most had SAH with high Hunt and Hess grades (III or IV). All had depressed level of consciousness; others had paresis (7/20, 35%), aphasia (1/20, 5%), and facial nerve palsy (1/20, 5%). Between 10 and 40 mg of nicardipine was used. A 60% increase in diameter of the main affected artery compared with the initial diameter measured in the initial angiographic run was achieved in all procedures. Clinical improvement (resolved focal symptoms or increased Glasgow Coma Score) occurred in 10 of 11 patients (91%). One patient died from complications of the initial hemorrhage. No complications occurred after 16 of 20 procedures (80%); minor complications without sequelae occurred after the remaining procedures. Follow-up of at least 2 months in 10 survivors revealed minor or no deficits in most patients with a Glasgow Outcome Score of 1 or 2 in 9 of 10 patients (90%).
CONCLUSION: In this small series, high-dose intra-arterial nicardipine infusion to treat SAH-associated vasospasm seems to be safe and effective.
Delayed cerebral ischemia as a result of vasospasm is a major cause of morbidity and mortality after aneurysmal subarachnoid hemorrhage (SAH).1 Between 4 and 14 days after SAH, approximately two thirds of the patients who undergo angiography will have some degree of vasospasm, and approximately one third will develop clinical vasospasm with severe restriction of cerebral blood flow and multiple brain infarctions.2
Although vasospasm refractory to medical therapy can be effectively treated by low-pressure balloon angioplasty, severe complications and limitations are associated with this technique, including the risk of vessel rupture.1 Moreover, its benefit is limited to the proximal circulation. Although intra-arterial infusion of papaverine has long been known to be at least transiently effective, severe adverse effects also have been reported, including paradoxical vasospasm, worsening ischemia, and profound neurologic deterioration.3
Beneficial effects have occurred in some patients with the use of intra-arterial infusion of a calcium-channel blocker (verapamil), with a low incidence of complications.4 Nicardipine is another calcium-channel blocker that has been used to treat vasospasm after SAH.5 The purpose of this study was to report our initial clinical experience with 11 patients treated with high-dose nicardipine for vasospasm after surgical treatment of aneurysmal SAH.
Patients and Techniques
Patients
The institutional review board at our facility approved this retrospective review of our experience with nicardipine in this patient population, with waiver of informed consent. From the logbook containing all interventional neuroradiology patients, we identified all patients with a diagnosis of aneurysmal SAH made between September 2003 and September 2004. We then accessed hospital information systems and identified those patients who developed clinically significant vasospasm refractory to medical treatment with “triple H” protocol therapy (hypervolemic, hypertensive, and hemodilution), had intra-arterial treatment with nicardipine, and did not undergo alternative therapy, (eg, balloon angioplasty or intra-arterial infusion of other pharmacologic agents). These patients comprised the study group.
Patients were suspected of having clinically significant vasospasm associated with aneurysmal SAH if they had depressed level of consciousness in combination with decreased Glasgow Coma Score (GCS) and focal neurologic deficits (eg, monoparesis, hemiparesis, aphasia, facial nerve palsy), within 14 days after surgical intervention (endovascular surgery or craniotomy and clipping), in the absence of other potential causes of these symptoms. All patients received prophylactic nimodipine after surgery. Intractable vasospasm was based upon 1) lack of response over a 2-hour period to triple-H therapy and 2) subsequent evidence of vasospasm on diagnostic angiography. Transcranial Doppler ultrasonography is not routinely obtained at our facility.
During the study period, we identified 31 patients who developed vasospasm after SAH. Twenty patients received triple-H therapy and showed some neurologic improvement. The remaining 11 patients showed no improvement with triple-H therapy and did not receive alternative therapy; all of these patients underwent angiography, showed evidence of vasospasm, and were treated with nicardipine. These 11 patients comprised the study group. The mean age was 57 years (range, 43–76 years); 10 of 11 patients were female (91%). GCS at admission was 3–6 in 3 of 11 patients (27%), 7–12 in 3 of 11 patients (27%), and 12–15 in 5 of 11 patients (45%).
All patients underwent CT without contrast and conventional angiography. Thirteen aneurysms were identified; 9 patients had a single aneurysm and 2 patients had 2 aneurysms. Most aneurysms were located in the anterior circulation: 3 in the posterior communicating artery, 3 in the middle cerebral artery bifurcation, 3 in the anterior communicating artery, and 1 in the internal carotid artery bifurcation. In the posterior circulation, there were 2 basilar artery summit aneurysms and 1 dissecting vertebral artery aneurysm (Table 1). Most patients presented with a Hunt and Hess (HH) grade of IV (n = 4) or III (n = 3) subarachnoid hemorrhages; only 4 patients were grade I or II. Two patients (18%) were treated with open surgery (craniotomy and clipping of the aneurysm) and 9 patients (82%) underwent endovascular surgery (aneurysm coil embolization). Endovascular surgery is the more prevalent technique of treatment in our institution. Each patient underwent a single surgical procedure.
Vasospasm site and nicardipine treatment data for study patients (n = 11) and procedures (n = 20)
Procedures
Nicardipine administration was performed under conscious sedation with intravenous administration of midazolam and fentanyl. Continuous blood pressure, heart rate, EKG, and Sao2 monitoring were performed. Intracranial pressure (ICP) monitoring was performed if a ventriculostomy or an ICP monitor was in place; otherwise, no evaluation of ICP during or after nicardipine infusion was obtained. Only 2 patients had a ventriculostomy in place for ICP monitoring. The ICP was continuously monitored during the procedure in these patients. No significant changes were noted; the initial ICP values were 9 and 10 mm Hg in the 2 patients. After catheter angiographic confirmation of vasospasm, the vessel showing the most severe narrowing within a vascular territory consistent with the new neurologic deficit was targeted for supraselective catheterization. After microcatheter placement, nicardipine at a concentration of 0.83 mg/mL in distilled water was infused in a 0.5–1-mL bolus over a 60-second interval. The dose rate was thus 0.415–0.83 mg/min. The infusion was titrated with the change in systolic blood pressure (SBP); if a severe drop in the SBP (>30% from SBP at start of infusion) was observed, the infusion was stopped and not resumed until SBP recovered to within 20% of the preinfusion level.
Postinfusion contrast injections were performed, obtained at 2–5-mg dose intervals to assess response. We based the decision to terminate infusion on the angiographic appearance of the vessels and did so when the most severely affected segment of the vessel recovered at least 60% of its normal diameter, measured in the most normal-appearing segment of the vessel and compared with baseline angiographic runs. This measurement was performed by the interventional neuroradiologist doing the procedure (J.C.C., S.K.L.) by comparing, side by side, the first and last angiographic images acquired, with the use of the flat screens of the bi-plane system Axiom Artis (Siemens Medical Systems, Erlangen, Germany) used to perform the procedures. No blinded observer measurements or internal corrections for magnification and specific measurements of multiple vessel segments, as described in previously published articles, were performed (Table 2)).3
Total nicardipine dose per treatment session and vascular territories treated
After therapy, the femoral sheath was left in place and was attached to an infusion pump. Heparinized saline (1000 U of heparin in 1 L of 0.9% saline) was infused at 30 mL/h to prevent thromboembolic complications to the lower extremities. The sheath was left in place for a maximum of 3 days; if no further treatment was needed, the sheath was removed and hemostasis achieved with manual compression or percutaneous suture of the arteriotomy site.
Data Analysis
From review of hospital records and follow-up clinic visits by 2 interventional neuroradiologists (J.G.T., R.A.T.), we documented basic demographic information, the location of the aneurysm(s), the HH grade of the subarachnoid hemorrhage, GCS at admission, and all surgical procedures. We then recorded the interval after surgery of the onset of vasospasm symptoms, the anatomic location of vasospasm, its severity (mild, moderate, severe), the dose of nicardipine used for treatment, SBP changes during treatment, and treatment-related complications. After therapy, we noted angiographic findings based on the observations at the time of treatment. We documented clinical findings (change in symptoms, change in GCS), all-cause mortality, presence of symptoms, and evidence of stroke on CT. GCS and Glasgow Outcome Score (GOS) at the last clinical follow-up visit were also noted; these were calculated from the medical records based on the evaluating physician notes. Clinical follow-up was performed by interventional neuroradiologists and the neurosurgeons at our institution, who were not blinded to the treatment received.
Results
Twenty cases of presumed postoperative vasospasm were identified in 11 patients; 1 case in 3 patients, 2 cases in 4 patients, and 3 cases in 3 patients. The onset of vasospasm was on day 4–8 postsurgery in 11 of 20 cases (55%), day 9–12 in 6 cases (30%), and day 13–15 in 3 cases (15%). Neurologic deficits included depressed level of consciousness (20/20 cases, 100%), paresis (7/20, 35%), aphasia (1/20, 5%), and facial nerve palsy (1/20, 5%). Vasospasm occurred in the M1 segment of the middle cerebral artery in 8 of 20 cases (45%), in the supraclinoid internal carotid artery in 4 cases (20%), in the A1 segment of the anterior communicating artery in 4 cases (20%), and in the A2 segment of the anterior communicating artery in 2 cases (10%). One case (5%) involved the M2–M3 segments of the middle cerebral artery and another (5%) involved the basilar artery and the posterior communicating arteries. The nicardipine treatment was performed in these affected territories. Twelve (60%) treatments were bilateral and 8 (40%) were unilateral according to the distribution of the compromise observed in the diagnostic angiogram. The degree of vasospasm was considered severe in 13 (65%), moderate in 6 (30%), and mild in 1 (5%) of the 20 cases.
Each vasospasm episode was treated with intra-arterial nicardipine infusion. In the 7 patients who received multiple nicardipine treatments, the mean interval between treatments was 2.7 days (Table 3). In 7 of 20 cases, 10–15 mg of nicardipine was used (35%); in 2 cases (10%), 16–20 mg; in 8 cases (40%), 21–25 mg; in 2 cases (10%), 31–35 mg; and in 1 case (5%), 36–40 mg. The procedure was well tolerated in all patients. No complications were observed in 16 of 20 cases (80%); minor complications without sequelae were observed in 4 cases (20%). These complications included thromboembolic events (n = 3) and acute transitory spasm of the middle cerebral artery (n = 1). In the patients who had a ventriculostomy in place, no changes in the ICP after the nicardipine infusion were observed. Blood pressures were fully documented in only 8 cases. Mean SBP was 180 (range, 150–201 mm Hg) at the beginning of the procedure and 148 (range, 75–192 mm Hg) at the end of the procedure. The SBP dropped 10%–15% (n = 3), 16%–20% (n = 1), 21%–25% (n = 1), 26%–30% (n = 2), and 31%–35% (n = 1) from the beginning to the end of the procedure.
Number of nicardipine treatments per patient and interval between treatments
The angiographic findings showed that the diameter of the most severely narrowed segment of the vessel increased to at least 60% of the normal diameter in all cases (Figs 1–4). No patients underwent additional surgical procedures after nicardipine therapy.
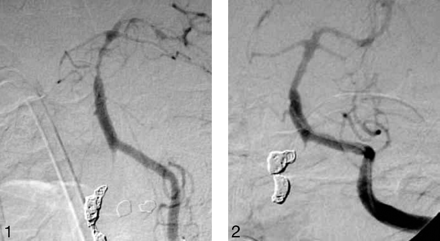
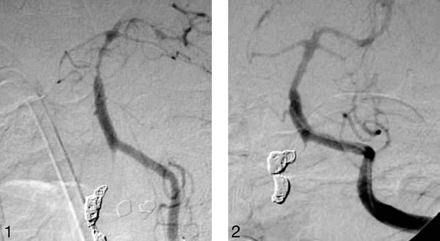
Left vertebral injection left anterior oblique (LAO) (A) and lateral (B) views demonstrate severe vasospasm of the distal basilar artery after SAH secondary to dissecting right vertebral aneurysm, treated with parent vessel occlusion with coils.
After a total 40 mg of intra-arterial nicardipine infusion (3 vascular territories injected). Almost total restoration of the vessel diameter is demonstrated.
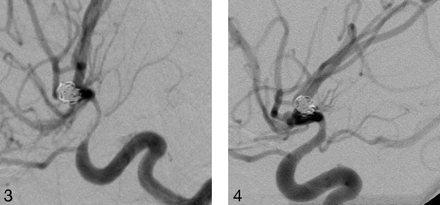
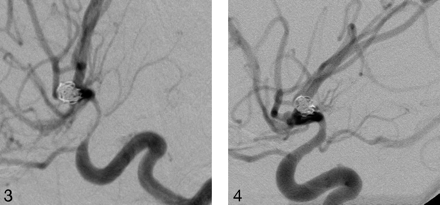
Right internal carotid artery (ICA) injection, lateral projection demonstrates severe vasospasm of the supraclinoid ICA after SAH.
After nicardipine infusion, there is more than 60% vessel diameter restoration.
After the last nicardipine infusion, clinical improvement with resolution of the focal symptoms and/or improvement in GCS was demonstrated in 10 of 11 patients (91%), all of whom were eventually discharged from the hospital. One of the 11 patients (9%) died 2 days after nicardipine infusion from complications related to the initial SAH.
Seven (70%) of 10 surviving patients had no evidence of stroke on CT imaging performed during the hospital stay. Only 3 patients (30%) had strokes demonstrated on CT.
Clinical follow-up was possible in 9 of the surviving 10 patients (90%) for between 2 and 8 months after hospital discharge (mean, 3.4 months); all had a GCS of 15. Five (50%) had a GOS of 1, 4 (40%) had a GOS of 2, and only 1 patient (10%) had a GOS of 3 (Table 3).
Discussion
There are limitations associated with balloon angioplasty for vasospasm treatment, including the potential risk of vessel rupture.1 Moreover, its benefit is limited to the proximal circulation. Intra-arterial infusion of papaverine has also been associated with severe adverse effects, including paradoxical vasospasm, worsening ischemia, and profound neurologic deterioration.3 For these reasons, there is a critical need to design and evaluate alternative therapies for this frequent complication of aneurysmal subarachnoid hemorrhage. Although the pathophysiologic mechanism of vasospasm after aneurysmal SAH is not entirely understood, there is evidence to suggest the efficacy of calcium antagonist therapy.6-9
Results of the use of new alternative medications, such as amrinone, have been reported.10 The reported experience with intra-arterial amrinone is limited to 2 patients, who demonstrated only mild improvement of the vasospasm and, moreover, were treated also with papaverine. Beneficial effects have also occurred in some patients with the use of intra-arterial infusion of a calcium-channel blocker (verapamil), with a low incidence of complications.4 However, the verapamil effect is transient, and neurologic improvement was noted in only 5 of 17 patients where verapamil was used as the sole treatment.
Nimodipine is a calcium antagonist that belongs to the chemical group of dihydropiridines. It has been used for many years as a drug therapy for SAH because it has been demonstrated to reduce the incidence of brain infarcts due to vasospasm by 34%–40% and to improve clinical outcome.11 However, nimodipine is not available in parenteral form in the United States. Preliminary experience with nimodipine has been reported.1 However, after nimodipine administration, clinical improvement was reported in only 76% of patients, and notable vascular dilation occurred in only 63%.
Nicardipine is also a dihydropiridine calcium-channel blocker and exerts its action more selectively on vascular smooth muscle than on cardiac muscle. Intravenous administration of nicardipine has demonstrated significant improvement in vasospasm, both angiographically and clinically, at a dose of 0.15 mg/kg/h.12 The efficacy of nicardipine has also been evaluated in the prophylaxis for vasospasm when administered intravenously at a dose of 0.075 mg/kg/h.13-15 Although the incidence of symptomatic vasospasm after subarachnoid hemorrhage was reduced, no improvement in overall outcome was noted at 3 months. Complications were reported as well, including prolonged hypotension, pulmonary edema, and renal dysfunction.15
Nicardipine is has also been used intra-arterially to treat vasospasm after SAH.5 A significant improvement in cerebral blood flow and mean transit time in ischemic regions in patients with SAH-induced vasospasm after intra-arterial nicardipine treatment has been reported when the patients were evaluated with cine CT perfusion.16
Intrathecal nicardipine prolonged release implants have also been used for vasospasm prophylaxis. These implants were placed at the time of the craniotomy for aneurysm clipping. However, the effectiveness of this technique is limited by rates of diffusion, and thus the implants only affect immediately adjacent vessels.17 Moreover, this technique can only be used in open surgery and not in endovascular surgery. Badjatia et al5 reported 42.1% improvement of posttreatment neurologic examination after intra-arterial infusion of nicardipine in postoperative vasospasm patients at a dose of 0.5–6 mg per vessel.
Because of the potential risks of balloon angioplasty and intra-arterial papaverine infusion for vasospasm treatment and the initial positive reports from the use of calcium-channel blockers, we began the use of intra-arterial infusion of nicardipine in these patients at our institution in September 2003.
Our study demonstrates benefits for most of the patients who underwent one or multiple sessions of high-dose intra-arterial vasospasm treatment with nicardipine, at a total dose of 10–40 mg per procedure. All but one patient improved significantly after treatment; in all other patients, the GCS score and/or focal deficits improved, and deficits completely resolved in some patients. Eighty percent of procedures had no complications, and complications resolved without sequelae after the remaining procedures. Although SBP dropped during all procedures, in most cases, this drop was less than 30%, and no clinical manifestations of this decrease were documented. The SBP returned to the pretreatment level quickly once the nicardipine infusion was terminated.
The angiographic results obtained showed at least 60% of vessel diameter restoration. Moreover, clinical follow-up demonstrated that most patients had a complete recovery to their baseline neurologic status, even after a catastrophic event such as a HH grade III-IV SAH.
Most of our patients demonstrated clinical recovery in their last clinic follow-up visit, as documented in the GCS and GOS assessments. This is encouraging considering the severity of the initial SAH.
The only deceased patient in our series was a very ill patient with a HH grade IV SAH from a ruptured aneurysm in the middle cerebral artery who died 2 days after admission from complications of the SAH. No worsening or improvement in the clinical status of this patient was observed after the nicardipine infusion. As no complications clearly associated with the intra-arterial infusion of nicardipine were noted in this patient, we do not believe that the endovascular procedure was related to the patient's final outcome.
However, as performed in the patients in our study, the benefit of nicardipine infusion was often transitory. Multiple treatment sessions were often needed to maintain the good response; 3 sessions were performed in 2 patients. Although this may be perceived as a limitation of intra-arterial nicardipine therapy for vasospasm, we believe nicardipine infusion can be repeated as many times as necessary, given the low rate of complications related to the procedure and the lack of adverse effects of the infusion.
Three thromboembolic complications were observed, but 2 were in the same patient in 2 different treatment sessions. We suspect that this outcome may reflect a prothrombotic condition in this patient. We certainly believe that the risks of nicardipine therapy, as performed in our study, are low compared with the deleterious consequences of untreated severe vasospasm.
Although a decrease in the blood pressure was documented in all patients, no change in ICP was observed in the 2 patients where this measurement was possible. The nicardipine infusion may be lengthy because of the infusion rate of 0.8 mg/min and the need to wait for the blood pressure to recover to continue the infusion. However, the average time per procedure was 1 hour, and it was considered technically easy to place the microcatheter in the ideal position and to infuse the drug. The length of the procedure was not thought to be physically demanding by the radiologists or the patients. All patients were anticoagulated with 5000 U of heparin at the beginning of the procedure to avoid the risk of thromboembolic complications.
We think that the higher nicardipine doses that we used may explain the difference between the 42.1% of improvement in neurologic status reported in other clinical series5 and the improvement in most of the patients who we found in our series documented with GCS and GOS.
Despite nicardipine treatment, a small number of patients had evidence of strokes on CT imaging. This might have been due to a delay in the clinical diagnosis of vasospasm but also might have been due to the transient effect of nicardipine infusion.
A limitation of our study was that we were not able to perform ICP monitoring during the procedure in most patients, because most did not have a ventriculostomy or an ICP monitor in place. Neurosurgery practice at our institution is that only patients who develop hydrocephalus because of the SAH undergo ventriculostomy. In the few patients who had a ventriculostomy in place, no changes in the ICP after the nicardipine infusion were observed, but ICP changes in the remaining patients could not be evaluated.
Conclusion
In this small series, the use of high-dose intra-arterial nicardipine infusions was safe and effective in the symptomatic vasospasm after aneurysmal SAH. Further studies are required to confirm these results in a larger population.
Acknowledgments
We acknowledge S. Gregory Jennings, MD, in the Department of Radiology at Indiana University for his assistance in editing the manuscript.
Footnotes
Current address for S.K.L.: Department of Radiology, University of Toronto, Toronto, Ontario, Canada.
References
- Received March 29, 2006.
- Accepted after revision August 11, 2006.
- Copyright © American Society of Neuroradiology