Abstract
BACKGROUND AND PURPOSE: In Alzheimer disease (AD), a peculiar regional cerebral blood flow (rCBF) abnormality has been reported in the posterior cingulate gyri and precunei, even at a very early stage. We performed a multicenter brain perfusion single-photon emission tomography (SPECT) study to evaluate the discrimination ability of an easy Z-score imaging system (eZIS) with a common normal data base between patients with very early AD at the stage of mild cognitive impairment and age-matched healthy controls.
MATERIALS AND METHODS: For a multicenter study, SPECT images of 40 patients with AD and 40 healthy volunteers were acquired from 4 gamma camera systems in 4 different institutions. Systematic differences of SPECT images between gamma cameras were corrected by using conversion maps calculated from the SPECT images of the same brain phantom. Receiver operating characteristic (ROC) analysis was performed to discriminate patients and controls by using a Z-score in the volume of interest (VOI), which had been defined as a region related to AD in subjects other than those in a multicenter study.
RESULTS: Bilateral posterior cingulate gyri, precunei, and parietal cortices were defined as a VOI showing rCBF reduction in very early AD. A new indicator of rCBF abnormality in the VOI provided 86% accuracy for distinction of AD and healthy controls in the multicenter study. The area under the ROC curve was 0.934.
CONCLUSION: Because an eZIS can use a common normal data base by converting site-specific SPECT data to the core data, the eZIS was useful for automated diagnosis of very early AD in routine studies in multiple institutions.
In the very early stage of Alzheimer disease (AD), even before a clinical diagnosis of probable AD is possible, decreases in regional cerebral blood flow (rCBF) and glucose metabolism in the posterior cingulate gyri, precunei, and parietal cortices have been reported by using positron-emission tomography (PET)1,2 or single-photon emission tomography (SPECT).3,4 We have already reported5 the superiority of the computer-assisted analysis of SPECT images using 3D stereotactic surface projection (3D-SSP) over visual inspection in the discrimination, from controls, of very early AD at the stage of mild cognitive impairment (MCI).6 However, this statistical approach requires a control data base for SPECT images. Even if one institution constructs a site-specific normal data base from the same camera and under the same processes for reconstruction of SPECT images, the transfer of this data base to other institutions is hampered by the physical characteristics of the SPECT cameras and collimators used. Despite the fact that the same types of cameras and collimators are used in several institutions, differences in the processes of reconstruction of SPECT images may also give rise to significant variations in the final SPECT images. To make it possible to share a normal data base in SPECT studies, we previously developed a new method using brain phantom experiments.7 In this method, a 3D conversion map was created from the division of 2 SPECT images of the same phantom obtained from the 2 different cameras after anatomic standardization by statistical parametric mapping (SPM).8 The conversion map was applied to convert an anatomically standardized SPECT image obtained from one camera to that from the other camera. This conversion algorithm was incorporated in a software program recently developed by us, easy Z-score imaging system (eZIS),9 for statistical analysis of SPECT images. The use of eZIS facilitates sharing of a normal data base of SPECT images in different institutions with different cameras.10 In the present study, we performed a multicenter SPECT study to evaluate the discrimination ability of eZIS with a common normal data base, between patients with very early AD and age-matched healthy volunteers, collected from different institutions by using different cameras.
Materials and Methods
Subjects for Determination of a Specific Region Showing rCBF Reduction in Very Early AD (Group 1)
Sixty healthy controls (28 men and 32 women; 54–83 years of age; mean, 71.5; SD, 8.3) and 29 patients with AD (13 men and 16 women; 57–85 years of age; mean, 70.9; SD, 7.8) at the stage of amnestic type of MCI, who showed progressive cognitive decline and eventually fulfilled the diagnosis of probable AD according to the National Institute of Neurologic and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association (NINCDS-ADRDA) criteria11 during the subsequent follow-up period of 2–6 years, were included in this study. Patients were recruited from those who complained of memory impairment in an outpatient memory clinic of the National Center Hospital for Mental, Nervous, and Muscular Disorders, National Center of Neurology and Psychiatry. At the first visit, they showed selective impairment in delayed recall (>1.5 SD below age-matched normal mean scores of a word-list learning test, a story-recall test, or the Rey-Osterrieth Complex Figure Test) in neuropsychologic examinations without apparent loss in general cognitive, behavioral, or functional status. They corresponded to 0.5 in the Clinical Dementia Rating (CDR).12 The Mini-Mental State Examination (MMSE)13 score ranged from 24 to 28 (mean, 25.8 ± 1.5) at the initial visit.
Control subjects were healthy volunteers without memory impairment or cognitive disorders who were recruited from the National Center Hospital for Mental, Nervous, and Muscular Disorders, National Center of Neurology and Psychiatry and whose MMSE scores ranged from 26 to 30 (mean, 28.5 ± 1.4). They did not differ significantly in age or education from the patients with AD.
Subjects for Automated Discrimination Between Very Early AD and Controls in a Multicenter Study (Group 2)
We retrospectively studied different subjects from group 1 comprising 40 patients with AD (15 men and 25 women; 51–83 years of age; mean, 71.0; SD, 8.5) at the stage of amnesic type of MCI, who also showed progressive cognitive decline and eventually fulfilled the diagnosis of probable AD according to NINCDS-ADRDA during the subsequent follow-up period of at least 2 years. They were recruited from patients who complained of memory impairment in an outpatient memory clinic of the Tokyo Metropolitan Ebara Hospital (18 patients); the National Center Hospital for Mental, Nervous, and Muscular Disorders, National Center of Neurology and Psychiatry (14 patients without duplication of patients with AD in group 1); the Juntendo University School of Medicine (7 patients); and the Fukujuji Hospital (1 patient). At the first visit, they showed selective impairment in delayed recall. They also corresponded to 0.5 in CDR. Their MMSE scores ranged from 24 to 28 (mean, 25.7 ± 1.6) at the initial visit.
Forty control subjects (18 men and 22 women; 50–83 years of age; mean, 71.0; SD, 0.3) were healthy volunteers without memory impairment or cognitive disorders. They were recruited from the Tokyo Metropolitan Ebara Hospital (17 subjects) and the National Center Hospital for Mental, Nervous, and Muscular Disorders, National Center of Neurology and Psychiatry (23 subjects without duplication of healthy subjects in group 1). Specifically, their performance was within normal limits (<1 SD) on both the Wechsler Memory Scale-Revised and the Wechsler Adult Intelligence Scale-Revised, and their MMSE score ranged from 26 to 30 (mean, 28.7 ± 1.5).
The local ethics committee approved this study for both healthy volunteers and AD subjects, all of whom gave their informed consent to participate. All subjects were right-handed, screened by a questionnaire regarding medical history, and excluded if they had neurologic, psychiatric, or medical conditions that could potentially affect the central nervous system, such as substance abuse or dependence, atypical headache, head trauma with loss of consciousness, asymptomatic or symptomatic cerebral infarction detected by T2-weighted MR imaging, hypertension, chronic lung disease, kidney disease, chronic hepatic disease, cancer, or diabetes mellitus.
Gamma Cameras
SPECT studies were performed on 4 gamma camera systems in 4 different institutions. The core dataset was acquired in the National Center Hospital for Mental, Nervous, and Muscular Disorders on a Multispect3 triple-headed camera (Siemens Medical Systems, Hoffman Estates, Ill) equipped with low-energy high-resolution fanbeam collimators (camera 1). Camera 2 in the Tokyo Metropolitan Ebara Hospital was a Prism 3000 triple-headed camera (Philips Medical Systems, Andover, Mass) equipped with low-energy high-resolution Fanbeam collimators. Camera 3 in the Juntendo University School of Medicine was a GCA9300A triple-headed camera (Toshiba Medical Systems, Tokyo, Japan) equipped with low-energy high-resolution Fanbeam collimators. Camera 4 in the Fukujuji Hospital was an E-CAM 2-headed camera (Toshiba Medical Systems) equipped with low-energy high-resolution Fanbeam collimators.
Before the SPECT scanning was performed, all subjects had an intravenous line established in all institutions. They were injected while lying down in the supine position with eyes closed in a dimly lit quiet room. Each subject received an intravenous injection of 600–740 MBq of technetium Tc99m ethyl cysteinate dimmer (Tc99m-ECD). Ten minutes after the injection of Tc99m-ECD, brain SPECT was performed. Preprocessing of projection data was performed by using a Hanning filter in camera 1 and a Butterworth filter in the other cameras. Reconstruction was performed by using a Shepp-Logan filter in camera 1 and a ramp filter in other cameras. Scatter correction was performed in cameras 1 and 3, but not in cameras 2 and 4. Attenuation correction was performed in cameras 1 and 2, but not in cameras 3 and 4. To share a normal data base, we performed Hoffman 3D Brain Phantom (Biodex Medical Systems, Shirley, NY) experiments by using these 4 cameras. The phantom was filled with 74 MBq of Tc99m pertechnetate, and SPECT data of the filled phantom were acquired and processed in the same manner as in the human brain study in each institution.
Determination of a Specific Region Showing rCBF Reduction in Very Early AD in Group 1
Voxel-based analysis for comparison of SPECT data of group 1 subjects between 60 controls and 29 patients with very early AD was performed by using Statistical Parametric Mapping 2 (SPM2) (Wellcome Department of Cognitive Neurology, London, UK) running on MATLAB (The MathWorks, Sherborn, Mass). The images were spatially normalized by using SPM2 to an original template for Tc99m-ECD.14 Then, images were smoothed with a gaussian kernel, 12 mm in full width at half maximum (FWHM). The processed images were analyzed by using SPM2. The effect of global differences in cerebral blood flow between scanning was removed by proportional scaling. The subject and covariate effects were estimated with the general linear model at each voxel. The resulting sets of t values constituted SPMs (SPM[t]). The SPM[t] were transformed to the unit normal distribution (SPM[Z]) and thresholded at P < .001 with multiple comparisons. A specific region showing rCBF reduction in very early AD determined by this SPM analysis was incorporated into the eZIS as a volume of interest (VOI).
eZIS Analysis in Group 2
An eZIS program9 for analysis of brain perfusion SPECT images was used in group 2 subjects to discriminate patients with very early AD at the MCI stage and age-matched healthy volunteers. Each SPECT image of the group 2 subjects after anatomic standardization by using SPM2 with an original Tc99m-ECD template followed by isotropic 12-mm smoothing was compared with the mean and SD of SPECT images of healthy controls that had already been incorporated in the eZIS program as a normal data base. Voxel-by-voxel Z-score analysis was performed after voxel normalization to global mean values: Z-score = [(control mean) − (individual value)]/(control SD). The normal SPECT data base of the eZIS program was constructed by using camera 1. It partially duplicated healthy controls in group 1 and comprised a middle-aged group (40–59 years of age, 19 men and 11 women), an older aged group (60–69 years of age, 18 men and 22 women), and an oldest aged group (70–86 years of age, 20 men and 20 women). The appropriate group of these 3 was used as a normal data base to match the age of a subject. The Z-score maps were displayed by overlay on tomographic sections and by projection with an averaged Z-score of 14-mm thickness to surface rendering of the anatomically standardized MR imaging template. A 3D conversion map calculated from phantom experiments7 yielded converted SPECT data from camera 2, camera 3, or camera 4 to the core SPECT data from camera 1. As a consequence, 43 of 80 SPECT data were converted to the core SPECT data.
Three indicators for characterizing rCBF decreases in patients with very early AD were determined: First, the severity of rCBF decrease in a specific region showing rCBF reduction in very early AD was obtained from the averaged positive Z-score in the VOI. Second, the extent of a region showing significant rCBF reduction in the VOI was obtained—that is, the percentage rate of the coordinates with a Z value exceeding the threshold value of 2. Third, the ratio of the extent of a region showing significant rCBF reduction in the VOI to the extent of a region showing significant rCBF reduction in the whole brain was obtained—that is, also the percentage rate of the coordinates with a Z value exceeding the threshold value of 2. This ratio indicates the specificity of the rCBF reduction in the VOI compared with that of the whole brain. These 3 indicators were obtained under 2 conditions, with or without data conversion to the core SPECT data by using phantom experiments.
Using the values of the 3 indicators as the threshold, we determined receiver operating characteristic (ROC) curves by using the ROCKIT 0.9β program developed by Metz et al (http://xray.bsd.uchicago.edu/krl).15 The program calculates the area under the ROC curves (Az), accuracy, sensitivity, and specificity. Accuracy was determined as the value at the point at which the sensitivity is the same as the specificity on the ROC curve. Then by using the PlotROC program (http://xray.bsd.uchicago.edu/krl), we also statistically calculated the interpolated values for drawing ROC curves.
Results
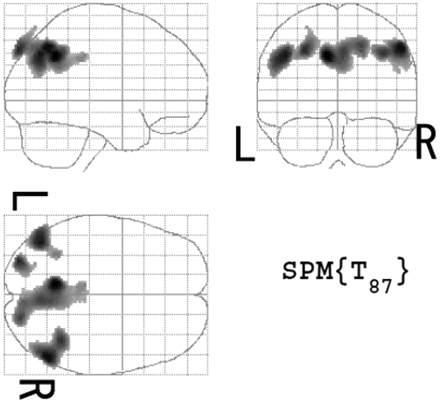
In group 1, the SPM2 analysis demonstrated significant declines of rCBF of patients with very early AD in the left (−8 −29 36, x y z; Z = 4.55) and the right (4 −29 35, x y z; Z = 4.21) posterior cingulate gyri, the left (−10 −54 38, x y z; Z = 6.1) and the right (6 −62 40, x y z; Z = 5.24) precunei, and the left (−44 −66 38, x y z; Z = 5.77) and the right (51 −58 45, x y z; Z = 5.91) inferior parietal cortices (Fig 1). These areas were registered to eZIS as a specific VOIs.
Maximum intensity projections of SPM2 results from group comparison of rCBF between patients with very early AD and age-matched healthy volunteers. Patients with very early AD showed significant decline of rCBF in bilateral posterior cingulate gyri, precunei, and inferior parietal cortices. Height threshold is <0.001, corrected for multiple comparisons.
With data conversion to the core SPECT data, 3 indicators for automated discrimination between patients with very early AD and healthy controls were obtained in group 2 subjects. Healthy controls and patients with AD showed 0.93 ± 0.31 and 1.63 ± 0.48 for severity, 6.32 ± 7.47% and 29.73 ± 18.72% for extent, and 1.84 ± 2.53 and 4.61 ± 2.52 for ratio, respectively. Significant differences (Student t test, P < .001) were obtained between patients with very early AD and healthy controls.
Without data conversion to the core SPECT data, healthy controls and patients with AD showed 0.85 ± 0.28 and 1.51 ± 0.41 for severity, 3.23 ± 4.07% and 22.06 ± 14.80% for extent, and 1.78 ± 3.00 and 4.68 ± 3.30 for ratio, respectively.
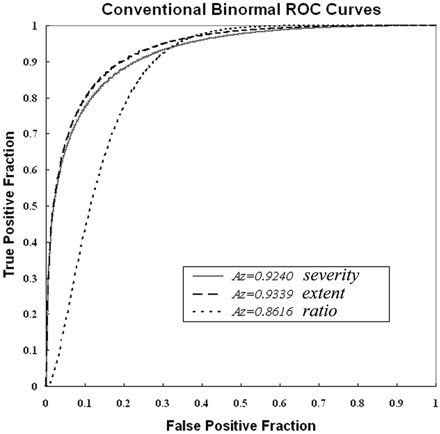
With data conversion to the core SPECT data, the ROC analysis showed Az values of 0.924, 0.934, and 0.862 for severity, extent, and ratio, respectively (Fig 2). Accuracies for discrimination between healthy controls and patients with very early AD were 85%, 86%, and 80%, respectively. Cutoff values for discrimination were 1.19, 14.2%, and 2.22 for severity, extent, and ratio, respectively.
ROC curves with data conversion to the core SPECT data for discrimination between patients with probable AD at the very early stage of MCI and age-matched healthy volunteers in a multicenter study by using the 3 indicators of severity, extent, and ratio.
Without data conversion to the core SPECT data, the ROC analysis showed Az values of 0.901, 0.911, and 0.828 for severity, extent, and ratio, respectively. Accuracies for discrimination between healthy controls and patients with very early AD also decreased to 82%, 84%, and 75%, respectively.
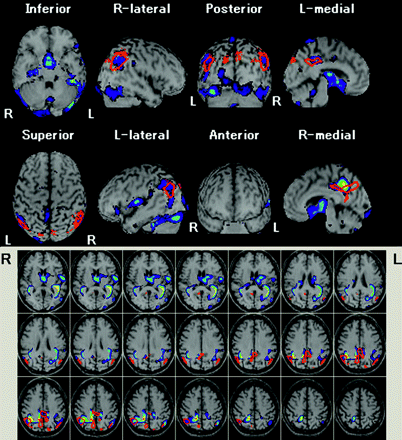
A representative case of a 78-year-old man with very early AD with an MMSE score of 24 is shown in Fig 3.
Automated voxel-by-voxel Z-score analysis by comparison of a brain perfusion SPECT image of a 78-year-old man with probable AD and an MMSE score of 24 with the mean and SD of SPECT images of healthy volunteers after normalization to global mean cerebral blood flow values. Color-scaled Z-score maps ranging from 2.0 to 5.0 with extent threshold of 300 voxels are displayed by overlaying on transaxial sections and surface rendering of the spatially normalized MR imaging template. Red lines enclose a VOI with the most significant decline of rCBF in very early AD obtained from group comparison with healthy volunteers by SPM2. The severity, extent, and ratio are 2.18, 77.2%, and 3.56, respectively.
Discussion
AD is the most common cause of progressive degenerative dementia that results in global cognitive deterioration, behavioral disturbances, and diffuse cortical atrophy associated with neuronal degeneration. The fact that recent medications like cholinesterase inhibitors delay the progression of AD has increased the importance of diagnosis of AD at the earliest possible stage. In terms of the early diagnosis of AD, the role of functional neuroimaging has become very important as a surrogate marker for the pathologic changes that underlie AD.
In our previous study on discrimination of patients with very early AD and age-matched healthy volunteers by using 3D-SSP analysis,5 the Az value in the posterior cingulate gyrus and precuneus obtained from ROC analysis was 0.937 with the reference region of global mean counts. The present result of 0.934 in the automated measurement of extent in the VOI was almost equal to that in the previous study. Considering that the number of patients with AD was almost twice that of the previous study and the SPECT images were collected from different institutions with different cameras, we believe that this Az value would be sufficiently high. This value also compared favorably with the Az value of 0.930 in a multicenter study using [18F]luorodeoxyglucose PET (FDG-PET)16 for discrimination of very mild AD using the MMSE scores of more than 24 from healthy controls. This comparable result between PET and SPECT might rather be expected despite the fact that superiority of PET over SPECT has been reported in detecting functional abnormalities in the AD brain.17,18 In direct comparison of statistical analysis of spatially normalized PET and SPECT scans in AD, Herholz et al19 reported that PET and SPECT provided comparable results for the main finding of temporoparietal and posterior cingulate functional impairment in mild-to-moderate AD, although the distinction between healthy volunteers and patients with AD is more robust and much less sensitive to threshold selection with PET than with SPECT.
In the present study, 3 indicators for characterizing rCBF decreases in very mild AD were developed. Mizumura et al20 argued that studying the extent of the region of abnormal rCBF that causes functional disorder was more rational than assessing the severity of the rCBF abnormality that reflected local tissue degeneration. Their contention may be supported by the fact that the discrimination power of the extent was slightly higher than that of the severity in the present study. The ratio indicating specificity of rCBF reduction in the VOI compared with the whole brain had the least discrimination power among the 3 indictors. This indicator may be useful for differentiation of AD from other neuropsychiatric diseases manifesting dementia. A further study will be necessary to confirm the utility of this indicator.
In the present study, conversion of 43 of 80 SPECT data to the core SPECT data for sharing of a normal data base raised the discrimination power of very early AD and healthy controls. Most of previous studies on the sharing of a normal data base have addressed only conversion of the reconstructed SPECT/PET counts in regions of interest from 1 physical condition to another.21–23 Spatially normalized images with a masking process as used in the present study were used in a recent report on sharing of the normal data base for FDG-PET.16 However, these reports have not converted the SPECT/PET image itself. Because SPECT exhibits greater variations in image quality among different centers than PET, conversion of SPECT images may be necessary for sharing of a normal data base. Degradation of image resolution to that of a worse image by spatial smoothing has been reported to be one of the most important factors for data sharing between different cameras.21–23 This adjustment was performed in the current study by smoothing all images with an isometric 12-mm FWHM gaussian kernel as described by Herholz et al.16
In the eZIS program, voxel-based analysis is performed by using a Z-score map calculated from comparison of a patient's data with the control data base in the same manner as in a 3D-SSP method.24 Anatomic standardization of SPECT images into a stereotactic space is performed by using SPM2. Therefore, this program is made from the combination of 3D-SSP and SPM2. It has been reported that 3D-SSP with 2D surface projection of cortical activities is less sensitive to artifacts derived from incomplete anatomic standardization of brain with localized cortical atrophy.25 However, a 3D-SSP technique loses information on the 3D location, which SPECT images inherently possess. This program also has an advantage of the capability of incorporation of SPM results into an automated analysis of Z-score values as a VOI. A specific VOI can be determined by group comparison of SPECT images of patients with a neuropsychiatric disease with those of healthy volunteers by using SPM.
Finally, we must refer to several study limitations. First, in the present study, other types of dementia, such as vascular- and frontotemporal-type dementia, were not included. However, previous reports demonstrated that the rCBF decrease in the posterior cingulate gyrus and precuneus is highly specific to AD.26,27 On the other hand, dementia with Lewy bodies (DLB) has been reported to show similar rCBF decrease in this area.28 Therefore, although the special emphasis on the decrease of rCBF in this area is reasonable for early diagnosis of AD, this finding may not exclude the possibility of DLB. Second, the onset age of AD was disregarded in the present study. Several studies with PET and SPECT have demonstrated differences in metabolic or rCBF abnormalities between an early onset and late onset of AD.29,30 Early-onset AD showed more severe metabolic or rCBF decreases in the posterior cingulate cortex, precunei, and parietal cortices than did late-onset AD. A further study should be undertaken to investigate the necessity of separate VOIs showing a rCBF decrease for early-onset and late-onset AD. Third, we retrospectively investigated amnestic patients with MCI who all converted to AD. The outcome for any patient with MCI is uncertain because many subjects may remain stable or even revert to a normal state, whereas others progress to dementia. Accordingly, the predictive study using this approach is much more important for MCI conversion to AD. In our previous study,31 converters from MCI to AD showed a wider area of decreased rCBF prominently in precunei and parietal cortices at the initial SPECT study than nonconverters. The present approach using new indicators for rCBF reductions in these areas would be quite useful for predicting outcomes of patients with MCI.
Conclusion
The automated analysis of brain perfusion SPECT by using eZIS with incorporation of a VOI related to very early AD revealed high performance in discriminating patients with very early AD at the stage of MCI and age-matched healthy volunteers. Moreover, a common normal data base is available in the eZIS by converting site-specific SPECT data to the core SPECT data by using the phantom study. Accordingly, the eZIS is very useful for diagnosing very early AD in a routine study in multiple institutions.
Acknowledgments
The authors thank the technical staff in our hospitals for data acquisition and John Gelblum for proofreading this manuscript.
References
- Received May 2, 2006.
- Accepted after revision June 14, 2006.
- Copyright © American Society of Neuroradiology