Abstract
BACKGROUND AND PURPOSE: Follow-up MR imaging examinations are increasingly used to monitor response to treatment in patients with spine infection. We aim to describe follow-up MR imaging examination findings 4–8 weeks after diagnosis and initiation of treatment of spine infections and to compare with clinical findings.
MATERIALS AND METHODS: Thirty-three patients with spinal infection and available baseline and 4–8-week follow-up MRIs were included in this retrospective cohort study. Baseline and follow-up MR imaging were graded by 2 neuroradiologists blinded to clinical characteristics and outcome. Clinical findings and outcomes were independently obtained by retrospective review of the medical record.
RESULTS: Compared with baseline MR imaging examinations, follow-up MR imaging more frequently demonstrated vertebral body loss of height (26/33 [79%] versus 14/33 [47%]; P < .001) and less frequently demonstrated epidural enhancement (19/32 [59%] versus 29/33 [88%]; P = .008), epidural canal abscess (3/32 [9%] versus 15/33 [45%]; P = .001), and epidural canal compromise (10/32 [31%] versus 19/33 [58%]; P = .008). Most follow-up MR imaging examinations demonstrated less paraspinal inflammation and less epidural enhancement compared with baseline. However, vertebral body enhancement, disk space enhancement, and bone marrow edema more often were equivocal or appeared worse compared with baseline. Twenty-one of 32 (66%) follow-up MR imaging examination overall grades were considered improved, 5 (16%) were equivocal, and 6 (19%) were worse. No single MR imaging finding was associated with clinical status.
CONCLUSION: Soft tissue findings, not bony findings, should be the focus of clinicians interpreting follow-up MR imaging results. No single MR imaging parameter was associated with the patients' clinical status.
MR imaging is the preferred imaging method of diagnosing vertebral disk space infections, vertebral osteomyelitis, and epidural abscesses (hereafter referred to as spine infections).1,2 Spine infections may cause serious, life-altering neurologic deficits, result in disabling pain, and occasionally lead to death. Therapy typically includes 4–8 weeks of pathogen-specific parenteral antimicrobials with or without surgical debridement.3 Patients with spinal infections are more often being managed nonsurgically in contemporary cohorts.4–6 After 4–8 weeks of parenteral therapy clinicians must decide whether to stop antimicrobial therapy altogether, extend parenteral therapy, begin oral therapy, or proceed with further diagnostic tests or surgical debridement. Serial imaging examinations, including MR imaging, are used to monitor therapeutic responses and guide clinical decisions, including whether to proceed with surgery, despite significant cost and scarce data on benefit.7
To our knowledge, 3 prior studies have described findings on follow-up MR imaging.7–9 Taken together, these reports suggest that some MR imaging findings may persist or worsen over time despite clinical improvement upon receipt of appropriate therapy. However, relatively small numbers of patients were included, and follow-up scans were performed over widely disparate times. Questions remain as to whether specific MR imaging findings correlate with clinical status, particularly during the 4–8 week follow-up period when clinicians often rely upon MR imaging findings to guide decision making.
The aim of this study was to describe the findings on follow-up MR imaging examinations during the clinically salient period 4–8 weeks after diagnosis and initiation of treatment of spine infections, and to correlate these follow-up MR imaging findings with clinical factors and patients' clinical status.
Materials and Methods
Study Design
This is a single-center retrospective cohort study. The institutional review board approved the study and waived patient informed consent for the minimal risk study in compliance with HIPAA regulations. Medical and surgical diagnostic approaches were performed at the discretion of the treating physicians. The patients involved in this study represent a subset of patients included in an article published previously that examined the long-term clinical outcome of patients with spine infection.10
Patient Population and Selection
One-hundred seventy-five consecutive cases of spine infection (disk space infections, vertebral osteomyelitis, epidural abscess) were identified from 1998 to 2002 after searching the Mayo Clinic Medical and Surgical Indexes,11 a radiologic data base, and a microbiology data base for related terms. All patients' clinical histories and diagnostic MR imaging were compatible with spine infection. Thirty-three patients met inclusion criteria: 1) age ≥18 years and 2) available baseline and 4–8-week follow-up MR imaging examinations (after initiation of therapy). The 142 patients who were not included either did not have follow-up MR imaging performed, follow-up MR imaging was not performed during the 4–8 weeks after initiation of therapy, or follow-up MR imaging was performed at another institution and was not available for review. Cases were confirmed by positive cultures (either blood or biopsies) or histopathologic findings (biopsy evidence of osteomyelitis or acute neutrophilic inflammation) in 32 of the patients; 1 patient was diagnosed with spine infection based upon suggestive clinical and radiographic findings alone. Granulomatous cases of spine infection (tuberculous, brucellosis, etc) were excluded because of their distinct clinical and radiographic findings compared with pyogenic spine infections.
Data Collection
A single investigator (T.J.K.), who was blinded to MR imaging interpretation, abstracted clinical data, management strategies, and outcome from all aspects of the inpatient and outpatient medical record using a coded data collection tool. Patients' clinical status at the time of follow-up MR imaging was recorded as improved (diminished back pain, resolution of systemic symptoms of infection such as fever, chills, etc), equivocal (back pain unchanged), or worse (increased back pain or persistent systemic symptoms of infection) based upon the treating physicians' comments and assessments during follow-up. When information was available, the clinical impact of the follow-up scan on the treating clinicians' therapeutic or diagnostic decisions was recorded. Patients were classified as having a systemic comorbidity if they were diagnosed with diabetes mellitus, systemic malignancy, chronic liver disease, had a serum creatinine ≥2.0 mg/dL, or had a history of radiation therapy that involved the spine. Patients were considered to have clinical failure based upon the treating clinicians' assessment. The presence of persistent neurologic deficits and the use of prescription pain medications at the time of last follow-up was recorded.
One of 2 neuroradiologists (K.F.L. and J.T.W.) reviewed each of the baseline and follow-up MR imaging scans blinded to patients' clinical information and recorded the results using a coded data collection tool. Specific parameters recorded included the anatomic level involved, the presence of disk space enhancement and T2 signal intensity, the presence of epidural enhancement, dimensions of epidural abscesses and percentage of epidural canal compromise, presence and dimensions of paraspinal abscesses, and presence and degree of paraspinal inflammation (mild, moderate, severe). An abnormality was considered an epidural abscess if there was a central area of nonenhancing T1 hypointensity surrounded by a rim of enhancement. When there was just nodular or linear epidural enhancement without a central area of hypointensity, it was considered epidural enhancement. The percentage of canal compromise was determined from axial images and described the percentage of compromise in the anteroposterior dimension of the spinal canal. The mild, moderate, and severe categories of paraspinal inflammation were graded subjectively based on the amount of soft tissue involved.
Recording the presence and extent of bony involvement was done in a graded fashion for bone marrow edema, vertebral body enhancement, and vertebral body loss of height using the following grading scale: no involvement, 1%–33% vertebrae involvement, 34%–65% vertebrae involvement, ≥66% vertebrae involvement. In addition to recording the presence or absence of a parameter on baseline and follow-up scans, investigators (K.F.L. or J.T.W.) assessed whether parameters looked improved, equivocal, or worse compared with the baseline MR imaging. Finally, investigators (K.F.L. or J.T.W.) assigned an overall assessment of improved, equivocal, or worse to each patient's follow-up scan compared with baseline based upon a comprehensive assessment of the degree and interval change in enhancement and inflammation in the disk space, paraspinal musculature, and epidural space. This assessment was a subjective assessment of the soft tissue response, mirroring interpretations used in clinical practice. The small sample size limits formal analysis of long-term outcomes and MR imaging findings.
MR Imaging Characteristics
All spine MRIs were performed on 1.5T GE Signa scanners (GE Healthcare, Little Chalfont, Buckinghamshire, UK) using a spine coil. Routine spine MR imaging technique for the work-up of suspected spinal infections included both T1-weighted (TR, 400–700 ms; TE, 14 ms) and fastspin-echo (FSE) T2-weighted (TR, 3000–5000 ms; TE, 105 ms) sequences. Before the administration of contrast, imaging of the involved spinal segment (ie, cervical, thoracic, and/or lumbar) was performed with T1 and T2-weighted imaging in the sagittal (512 × 256 matrix with a NEX of 2 [T1] or 4 [T2]) and axial (320 × 256 matrix with a NEX of 2 [T1] or 3 [T2]) planes. Sagittal imaging was performed at a section thickness of 4 mm and a section gap of 1 mm while axial imaging was performed with 5-mm thick sections and no gap. Intravenous gadolinium was given for all suspected spinal infections. The spine was then imaged in the axial and/or sagittal plane after contrast with T1-weighted sequences. Twenty-four of 33 patients' follow-up MRIs used a postcontrast T1 fat saturation technique. All MR imaging examinations were assessed to allow for appropriate changes in the scan parameters for each patient (ie, enlarging the field of view, adding additional sequences, etc). One patient in the study did not receive contrast on the follow-up MR imaging because patient motion prompted early termination of the examination, limiting evaluation of epidural, paraspinal, and disk space enhancement.
Statistical Analysis
To determine differences between baseline and follow-up MR imaging findings, the Wilcoxon signed rank test was used for ordinal variables and the McNemar test for categoric variables. To determine MR imaging findings that best correlate with clinical status (and presumably resolution of infection as well), we compared follow-up MR imaging findings from successfully treated patients with clinical improvement at follow-up with patients in whom therapy was unsuccessful or who were not clinically improved at follow-up. For this comparison, Fisher exact test was used for categoric variables, and the Wilcoxon rank sum test was used for ordinal variables. To determine independent host, microbiologic, clinical, management, and outcome factors associated with MRIs that were overall assessed as improved versus equivocal or worse, Fisher exact test was used. All analyses were also performed excluding patients who had surgery performed to assess for any confounding surgery may have introduced into the results. A 2-sided P-value of ≤0.05 was considered significant. Data were analyzed using JMP (5.1.2; SAS Institute, Cary, NC).
Results
Clinical Characteristics and Management
Thirty-seven noncontiguous loci of spine infection in the 33 patients included in the study were evaluated. Patient characteristics are presented in Table 1. It is noteworthy that only 1 patient was known to be an intravenous drug user, and none were infected with the human immunodeficiency virus. Staphylococcus aureus was the most frequent isolate (45%). The median duration of symptoms before diagnostic MR imaging was 22 days (range, 7–162 days). A median of 3 MR imaging scans was performed in each patient, with 1 at baseline and 1 4–8 weeks after initiation of therapy in accordance with inclusion criteria. Follow-up MR imaging was performed a median of 44 (range 29–63) days after diagnostic MR imaging. Only 12 patients had follow-up erythrocyte sedimentation rate (ESR) data available, which limited the formal assessment of ESR on clinical outcomes. Of these, 4 patients had ESR values that remained markedly elevated or had increased from baseline. One patient's MR imaging was read as improved, 2 patients' MRIs were read as equivocal, and 1 patient's was worse.
Demographic and clinical characteristics
Five of 33 patients underwent surgery as part of their initial treatment. All 33 patients were treated with either parenteral or highly bioavailable oral antimicrobial therapy for a median duration of 44 days (range, 28–116 days). Thirteen patients received oral antimicrobials after parenteral therapy for a median additional duration of 42 days (range, 4–201 days).
Long-Term Clinical Outcomes
Patients were followed for a median duration of 651 days (range 70–2102). In 3 patients, clinical treatment was unsuccessful: 2 patients' follow-up MR imaging was graded worse, and 1 improved. The small number of treatment failures precludes statistical analysis to determine radiographic features associated with treatment failure. Five (15%) patients were left with neurologic deficits related to their spinal infection at the time of last evaluation. No patient went on to develop new neurologic deficits after the 4–8 week clinical follow-up. Eleven (33%) patients continued to take prescription pain medications for back pain at the time of last evaluation.
MR Imaging Findings
The presence or absence of specific findings at baseline and follow-up MR imaging is shown in Table 2. Follow-up MRIs more often demonstrated vertebral body loss of height compared with diagnostic scans (P < .001) and less often demonstrated epidural enhancement (P = .008), spinal canal abscess (P < .001), and a 25% compression of the spinal canal (P = .008). Other differences in the presence or absence of parameters on baseline versus follow-up MR imaging did not reach statistical significance (Table 2). Of the patients with epidural abscesses, the size of the abscess (measured at maximum diameter in cross-section) ranged from 5 to 18 mm. Four patients had spinal cord edema (3 had thoracic lesions, 1 had a cervical lesion), 3 of whom presented with paresis.
The presence of specific MR imaging findings at baseline and follow-up MR imaging
The descriptive assessment (ie, improved, equivocal, worse) of interval changes in specific MR imaging findings on follow-up compared with baseline images is shown in Table 3. Most patients showed improvement in paraspinal inflammation (16/32) and epidural enhancement (21/32). Among the 17 patients with epidural canal abscesses present at baseline, 15 were assessed as improved at follow-up MR imaging. In contrast, disk space enhancement, bone marrow edema, and vertebral enhancement were more likely to be assessed as equivocal or worse (Table 3). Figure 1 demonstrates typical temporal changes observed.
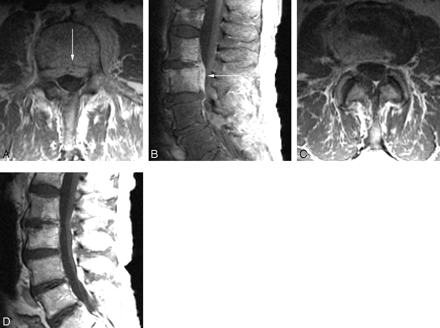
Baseline postcontrast axial T1 (A) and sagittal T1 fat-saturated (B) images demonstrate enhancing tissue in the ventral epidural space (arrows) as well as enhancement in the L3 and L4 vertebral bodies. Follow-up axial (C) and sagittal (D) postcontrast T1-weighted images demonstrate resolution of the enhancing epidural tissue. Persistent enhancement of the vertebral bodies and disk space is seen in this patient despite clinical improvement.
Assessment of interval changes of specific MR imaging findings at follow-up MR imaging compared with baseline MR imaging
To determine MR imaging findings that best correlate with clinical status (and presumably resolution of infection as well), we compared follow-up MR imaging findings from successfully treated patients with clinical improvement at follow-up (resolution of systemic signs and/or symptoms of infection and improved back pain) with patients for whom therapy failed or who were not clinically improved at follow-up. The results are shown in Table 4. No single MR imaging characteristic differentiated the groups.
The presence of specific follow-up MR imaging findings by clinical status
The overall assessment of MR imaging response, clinical status, and the clinical impact of the scan is shown in Table 5. Twenty-one of 32 (66%) patients' follow-up MRIs looked improved when graded according to comprehensive soft tissue findings (epidural enhancement, paraspinal inflammation, and disk space enhancement). Twenty-five of 32 (76%) patients demonstrated clinical improvement by the time of follow-up MR imaging. Of the 6 patients whose follow-up MR imaging was graded worse at follow-up, 5 were nonetheless clinically improved after nonsurgical treatment with antimicrobials. Figures 2 and 3 are illustrative. Six of 11 (55%) patients whose MR imaging was equivocal or worse consequently had their antibiotic course prolonged or an invasive intervention compared with 7 of 21 (33%) patients with improvement on follow-up MR imaging.
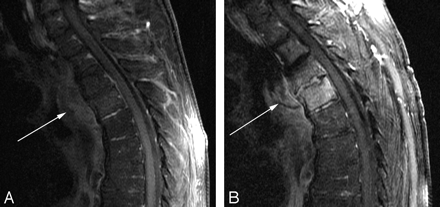
Baseline postcontrast fat-saturated sagittal (A) T1-weighted imaging demonstrates abnormal enhancement of the paraspinal soft tissues (arrow). Follow-up postcontrast T1-weighted imaging (B) demonstrates persistence of paraspinal enhancement (arrow) and new enhancement in the vertebral bodies and disk space. Despite a worsening appearance on MR imaging, this patient was improving clinically.
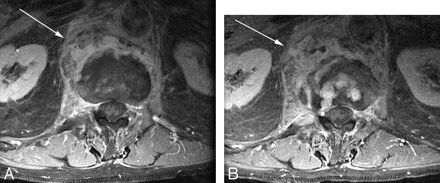
Baseline postcontrast fat-saturated axial T1-weighted imaging (A) demonstrates a paraspinal phlegmon (arrow). Despite clinical improvement, follow-up postcontrast T1-weighted images demonstrate apparent worsening. The paraspinal phlegmon has increased in size (arrow) and now has a small nonenhancing component suggestive of early abscess formation. There is also increased enhancement in the disk space.
Overall follow-up MR imaging results by clinical status and clinical impact
Associations between various host, pathogen, clinical, and medical and surgical management factors with follow-up MR imaging scans assessed as improved versus equivocal or worse are shown in Table 6. There was a trend toward more patients <75 years old having improvement on follow-up MR imaging (P = .06). Fifty-five percent of patients with equivocal or worse follow-up MRIs had systemic comorbidities, compared with 24% of patients with improvement on follow-up MR imaging (P = .12). Six of 11 (55%) patients with equivocal or worse MR imaging were treated with additional oral antimicrobials versus 7 of 21 (33%) with improved MR imaging (P = .28). All 5 patients with culture-negative infections had improvement on follow-up MR imaging (P = .14). There were no significant differences in duration of antimicrobial therapy or the use of additional oral antimicrobial therapy between patients with improved or nonimproved follow-up MR imaging.
Clinical characteristics and patient outcomes by overall follow-up MR imaging results based upon epidural, paravertebral, and T2 disk space changes
We assessed whether initial surgical management affected the results by performing all analysis excluding those patients who were treated with surgery. In no instance did an association differ across the statistical significance level of .05 when analyzed excluding patients managed surgically. Among the 5 patients who were managed surgically, 1 could not be given an overall assessment because no contrast was administered at follow-up examination. The remaining 4 patients were all graded as improved at follow-up examination compared with baseline examination.
Discussion
This study represents a review of serial MR imaging studies among patients with spinal infections and illuminates typical MR imaging characteristics on follow-up examinations during the clinically relevant 4–8-week follow-up period. These results, in context with other results, should aid in the interpretation of follow-up MR imaging among patients with spinal infections.
Imaging characteristics on follow-up MR imaging were notable in that disk space enhancement, paraspinal inflammation, vertebral body enhancement, marrow edema, and compression fractures were present in similar or greater proportions of participants at follow-up than at diagnostic MR imaging. Paraspinal abscesses, epidural canal abscesses, epidural enhancement, and T2 disk space abnormalities tended to improve or resolve on follow-up MR imaging. These findings are supportive of those by Gillams et al8 and Veillard et al.9 In Gillams et al,8 diminished soft tissue inflammation in 8 of 14 patients (57%) was one of the first signs of improvement. Among patients with soft tissue improvement, however, most in their study demonstrated radiographic worsening in the disk space or bone, or they developed new anatomic areas of involvement. Gadolinium enhancement was noted to persist, albeit at reduced intensity, for a median of more than 4 months and completely resolved in only 1 follow-up MR imaging done after more than 2 years. Veillard et al9 noted improvement in most epidural and paravertebral abscesses in 16 patients with MR imaging performed 1 month after diagnosis. Numaguchi et al12 and Sadato et al13 each described persistence of contrast enhancement in patients despite clinical improvement. Our study found similar results. It is noteworthy that most patients continued to have some degree of soft tissue inflammatory changes at the time of follow-up MR imaging despite clinical improvement.
There were no statistically significant differences between the subgroup of successfully treated patients with clinical improvement at follow-up compared with patients in whom therapy was unsuccessful or who were not clinically improved at follow-up. All patients in the unfavorable clinical status category had vertebral compression fractures. It remains possible that differences exist between MR imaging findings of clinically improved patients and clinically unimproved patients. A larger sample size may increase the power of a future study to detect such differences. However, the clinical utility of such differences would be questionable. For instance, T2 disk space abnormalities were present in 65% of the group with favorable clinical parameters compared with 90% with unfavorable clinical parameters. If these proportions remain approximately stable, a larger sample size may demonstrate statistical significance, but patients in either group would still be more likely to have abnormalities than not. The parameter's discriminatory ability would likely be of limited value to clinicians.
In contrast to our study, Carragee7 found that follow-up MR imaging within 6 weeks of diagnosis demonstrated overall worsening in 7 of 8 patients, often despite clinical improvement. However, 4 of these 8 patients were imaged less than 4 weeks after diagnosis, and no distinction was made between changes seen in bone, disk space, paraspinal tissues, or the epidural canal. Our criteria for the timing of examinations and basis of the overall grading solely on soft tissue parameters probably contributed to the differing findings.
We note that only 1 of 21 patients with improved scans at follow-up went on to experience treatment failure. Thus, like Carragee's findings for ESR values7 used for similar purposes, a positive response predicts good outcomes.14 However, with both serial ESR values and MR imaging examinations, most patients who show no improvement on these tests are still likely to have a good clinical outcome, highlighting the poor specificity of each test for the purpose of predicting treatment failure.
The exact pathophysiologic condition in a treated spine infection that results in the MR imaging signal intensity patterns that we have described (such as why bone marrow edema and enhancement were more often present on follow-up than baseline MR imaging) remains unclear. One hypothesis may be a greater vascular supply and increasing granulation tissue associated with healing results in this finding. The development of loss of vertebral height probably represents bone collapse related to pathologic destruction from the infection. The collapse itself may be primarily a mechanical phenomenon that does not imply untreated or uncontrolled infection but instead represents the sequelae of an appropriately treated and eradicated infection in many instances.
Limitations in the study include its retrospective nature and small sample size. The latter is a function of the low incidence of disease. Our study is not adequately powered to ruleout further differences between baseline and follow-up MR imaging findings and between various clinical parameters and follow-up MR imaging. However, given the findings presented, it is unlikely that a single radiographic finding will emerge that portends clinically relevant information. Selection bias may have affected results (ie, patients who underwent follow-up scans may be different from those who did not). This cohort may represent a sicker group of patients than those who did not get scans. In addition, a relatively high proportion of cases in the study were culture-negative, probably because in such cases MR imaging is used more liberally to monitor response to empiric therapy. The small sample size limits our ability to assess the ability of ESR and MR imaging findings on long-term outcomes. Finally, no formal assessment of interobserver or intraobserver reliability of the radiologists' interpretations was performed.
Conclusion
Despite the increasing use of follow-up MR imaging to monitor response to treatment in patients with spine infection, the clinical utility of this strategy has not been demonstrated. We demonstrated no specific associations between follow-up MR imaging findings and clinical status in this cohort of patients with spine infections, though small sample size may limit our ability to do so. We do describe in detail follow-up MR imaging findings during a clinically relevant period, which may serve as a guide for interpreting scans in the future. This study does not support the routine use of follow-up MR imaging in patients who are clinically responding to therapy.
References
- Received March 27, 2006.
- Accepted after revision June 13, 2006.
- Copyright © American Society of Neuroradiology