Abstract
SUMMARY: Wolfram syndrome is a rare autosomal recessive disorder featuring diabetes insipidus, diabetes mellitus, optic atrophy, and deafness; DIDMOAD is a commonly accepted anonym for this disorder. We describe a 35-year-old man with Wolfram syndrome, who had marked atrophy of the brain stem, middle cerebellar peduncle, and cerebellum. Despite these MR imaging findings involving the pontocerebellar tract, the patient had no neurologic abnormalities suggesting dysfunction of the brain stem or cerebellum. Patients with Wolfram syndrome may have discrepancies between neurologic and radiologic findings.
In 1938, Wolfram and Wagener1 described a family in which 4 siblings developed bilateral optic atrophy and diabetes mellitus, followed by deafness, incontinence, and ataxia. The frequent association of diabetes insipidus, diabetes mellitus, optic atrophy, and deafness led to the acronym DIDMOAD, indicating 4 cardinal features.2 DIDMOAD, commonly known as Wolfram syndrome, is a rare autosomal recessive disorder with demonstrable clinical and genetic heterogeneity,2–4 and a gene for Wolfram syndrome (WFS1) has been cloned and mapped to chromosome 4p.5 The onset of Wolfram syndrome is usually juvenile, and most patients are referred to pediatricians or endocrinologists. Therefore, despite the striking neurologic and neuroradiologic features of Wolfram syndrome, the disorder has made little impact on the neurologic and neuroradiologic literature. In the present report, we describe a patient with Wolfram syndrome presenting marked MR imaging abnormalities with few neurologic abnormalities.
Case Report
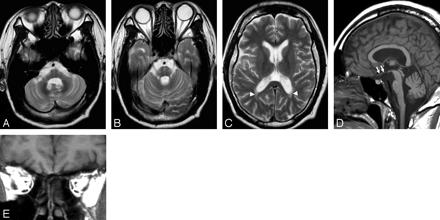
A 35-year-old man had diabetes insipidus, diabetes mellitus, and visual loss due to optic atrophy since he was 3 years of age. He had been diagnosed with Wolfram syndrome on the basis of the typical clinical features and familial history of diabetes mellitus. The man was admitted to our hospital because of consciousness disturbance in June 2005. Laboratory examinations showed diabetic ketoacidosis. He was stuporous and opened his eyes only to painful stimuli. After the patient recovered from diabetic ketoacidosis, neurologic examinations revealed complete visual loss and mild sensory hearing loss, but there were no findings suggesting abnormalities of the brain stem or cerebellum, such as nystagmus, dysarthria, or cerebellar ataxia; however brain MR imaging showed moderate atrophy of the brain stem and middle cerebellar peduncle and mild atrophy of the cerebellum (Fig 1A, -B), in addition to T2 elongation of peritrigonal white matter and absence of the T1 hyperintensity normally recognized in the posterior pituitary lobe (Fig 1C, -D). There was atrophy of intraorbital and intracranial optic nerves and tracts (Fig 1D, -E). The patient was treated by intensive insulin therapy and discharged 3 weeks later. Diabetic mellitus was well controlled, and his neurologic status showed no obvious deterioration in January 2006.
Axial T2-weighted MR images show atrophy of the brain stem and middle cerebellar peduncles and areas of elevated signal intensity in the pons (A and B). Cerebellar atrophy is also recognized. Signal-intensity abnormalities in optic radiation on both sides are also seen (C, arrowheads). A sagittal T1-weighted MR image shows intracranial optic nerve atrophy (D, arrows) and absence of T1-hyperinensity normally recognized in the posterior pituitary lobe. A coronal T1-weighted image shows atrophy of both intraorbital optic nerves (E).
Discussion
Our patient had elevated signals in the pontine base and marked atrophy of the brain stem, middle cerebellar peduncle, and cerebellum. These changes involving the pontocerebellar tract resembled MR imaging findings in multiple system atrophy (MSA) and some kind of familial spinocerebellar degeneration (eg, spinocerebellar ataxia [SCA] 1, SCA2, Machado-Joseph disease [MJD or SCA3], or dentatorubral-pallidoluysian atrophy [DRPLA]). Neuroradiologically, MSA features pontocerebellar atrophy and pontine signal-intensity changes widely known as “cross signs.” It is difficult to radiologically differentiate the present case from MSA, but the age at onset of MSA is usually the 5th or 6th decade. The patients with SCA1 have brain stem and cerebellar volume loss and mild supratentorial generalized volume loss.6,7 Patients with SCA2 have more severe olivopontocerebellar atrophy than patients with SCA1 and SCA3, and they also have supratentorial atrophy.7,8 The patients with SCA3 and DRPLA have atrophy of the superior cerebellar peduncle, in addition to pontocerebellar atrophy.7,9–11 In patients with DRPLA, T2-weighted images usually show white matter hyperintensities. In our patient, there was no atrophy of the superior cerebellar peduncle, and white matter hyperintensities were more focal than those in patients with DRPLA.
Although it is difficult to radiologically differentiate the present case from the previously mentioned pontocerebellar disorders, our patient had no neurologic abnormalities suggesting dysfunction of the brain stem or cerebellum. Previous reports of Wolfram syndrome2,12–14 showed nearly the same MR imaging findings as those of our patient, but the previous patients had some neurologic deficits secondary to brain stem and cerebellar atrophy.2,13 For example, a patient described by Scolding et al2 had pendular nystagmus in the primary position and gaze-evoked nystagmus as a vestibulocerebellar dysfunction, and another patient had slurring dysarthria and limb ataxia, which corresponded with pontocerebellar atrophy. A patient reported by Pakdemirli et al13 had dysarthria, and her brain MR imaging showed atrophy of the brain stem and cerebellum. In a pathologically confirmed case of Wolfram syndrome in a patient described by Genís et al,15 the patient had slight dysarthria and gait unsteadiness caused by atrophy of the pons and cerebellar white matter; however, some previous reports of Wolfram syndrome did not contain sufficient description of the neurologic conditions.12,14
In our patient, it is unclear why the neurologic deficits secondary to brain stem and cerebellar atrophy were absent despite apparent MR imaging abnormalities in that region. A previous neuropathologic report of Wolfram syndrome15 showed moderate loss of neurons in pontine nuclei, inferior olives, and dentate nuclei of the cerebellum, and cerebellar white matter that was reduced in size without demyelination. Purkinje cells were reduced and showed axonal ballooning, but the granular cell layer was normal. Similarly, in our patient, the atrophy was more apparent in the brain stem and middle cerebellar peduncle than in the cerebellar cortices. This relative preservation of cerebellar cortical architecture might be one of the causes of the neurologic normality of cerebellar function, which was mismatched with brain stem and cerebellar atrophy on MR imaging. In the previous reports of Wolfram syndrome,2,13 the patients had some neurologic deficits secondary to brain stem and cerebellar atrophy, but these deficits were considered milder than those in patients with multiple system atrophy.
Additionally, our patient had T2 elongation of peritrigonal white matter and absence of T1 hypeintensity normally seen in the posterior pituitary lobe. The former could reflect primary or secondary degeneration of optic radiation, because past microscopic examinations showed atrophy of optic nerves, chiasm, tracts, and optic radiations and neuronal loss in the lateral geniculate nuclei and superior colliculi.15 The latter suggested loss of vasopressin-containing neurons as a cause of diabetes insipidus.
In summary, in patients with Wolfram syndrome, there were marked brain MR imaging abnormalities affecting the pontocerebellar tract, whereas there were few neurologic abnormalities suggesting cerebellar dysfunction. These patients may have discrepancies between neurologic and radiologic findings.
Acknowledgments
We thank Drs. Ichiro Tatsuno and Kenichi Sakurai in the Department of Clinical Cell Biology, Graduate School of Medicine, Chiba University, for their great contributions to diagnosis and treatment of the patients.
References
- Received January 13, 2006.
- Accepted after revision February 17, 2006.
- Copyright © American Society of Neuroradiology