Abstract
BACKGROUND: Recent studies have focused on mechanical thrombectomy as a means to reduce the time required for revascularization and increase the revascularization rate in acute stroke. To date no systematic evaluation has been made of the different mechanical devices in this novel and fast-developing field of endovascular interventions. To facilitate such evaluations, we developed a specific in vivo model for mechanical thrombectomy that allows visualization of dislocation or fragmentation of the thrombus during angiographic manipulation.
METHODS: Angiography and embolization with a preformed thrombus was performed in 8 swine. The thrombus was generated by mixing 25 IU bovine thrombin and 10 mL autologous blood. For visualization during angiography, 1 g barium sulfate was added.
RESULTS: The preformed thrombus exhibited mechanical stability, reproducibility, and high radiographic absorption, providing excellent visibility during angiography. The setting allowed selective embolization of targeted vessels without thrombus fragmentation. Despite the application of barium sulfate no local or systemic reaction occurred. Histologic evaluation revealed no intimal damage caused by the thrombus or contrast agent washout.
CONCLUSION: The model presented here allows selective and reliable thromboembolization of vessels that reproduce the anatomic and hemodynamic situation in acute cerebrovascular stroke. It permits visualization of the thrombus during angiography and intervention, providing unique insight into the behavior of both thrombus and device, which is potentially useful in the development and evaluation of mechanical clot retrieval in acute cerebrovascular stroke.
Acute ischemic cerebrovascular stroke remains a severe disease despite improved outcome following intra-arterial thrombolysis (IAT).1,2 The outcome depends largely on the length of time between onset of symptoms and revascularization, the recanalization rate, and whether intracranial hemorrhage occurs.3-7 Furthermore, the time frame for effective IAT is limited.1,2 Recent studies have examined whether mechanical thrombectomy can accelerate revascularization and increase the revascularization rate. Most of these studies, however, relied on in vitro experiments8,9 or primarily clinical data.10-17 Some authors report a benefit from mechanical thrombectomy compared with thrombolysis.9,14,15,18
Numerous devices have been introduced to the technique of endovascular intervention. No study, however, has yet systematically evaluated and compared the various mechanical thrombectomy devices available for neurointervention. Ideally, such an evaluation requires a specific model that permits selective thromboembolization of the targeted vessel and that is large enough for the evaluation of standard-sized devices. Particularly with regard to mechanical thrombectomy the model should allow visualization of dislocation or fragmentation of the thrombus during angiographic manipulation, which has not been provided by any animal model so far.
The aim of the present study was to develop and evaluate such an in vivo animal model dedicated to the evaluation of mechanical thrombectomy devices used to treat acute cerebrovascular stroke.
Methods
Animal Care
All procedures were conducted according to international guidelines and were approved by the responsible local authorities. Eight swine ranging in weight from 43 to 47 kg were used in this study. Free access to food and water was given until the night before angiography. Sedation was induced by 0.05 mg/kg atropine and 15 mg/kg midazolam, and endotracheal intubation performed. The general anesthesia was maintained by 2% isoflurane inhalant. Vital parameters such as arterial blood pressure, heart rate, and expired oxygen and carbon dioxide levels were continuously recorded. The expired carbon dioxide levels were kept between 30 and 35 mm Hg. After the experiments the animals were euthanized with an intravenous injection of 20 mmol potassium chloride.
Thrombus Preparation
The blood clot for selective thromboembolization was prepared by mixing autologous venous blood with bovine thrombin (Dade; Dade Behring, Newark, Del). First a dilution series by using different amounts of thrombin mixed with 10 mL blood in a syringe was performed to assess the adequate thrombin concentration. Best results with regard to reproducibility and stability of the thrombus were achieved with 25 IU per 10 mL (2.5 IU/mL). After mixing for 10 seconds, the blood was injected into a silicone tube (3-mm inner diameter, 1000 mm long with injection-connection-piece; DIN 58362-VL-P, Clinico Medical, Bad Hersfeld, Germany). The incubation time was 60 minutes at room temperature. The generated whole blood or red thrombus was then washed in physiologic saline solution and incubated for a further 20 minutes before application.
Contrasted Thrombus Preparation
To increase radiographic absorption of the thrombus, a second dilution series with application of 3 different contrast agents was performed. In series 1, different amounts of barium sulfate; in series 2, different amounts of tantalum (Tantalum dust, Cook, Bloomington, Ind) were placed in the syringe before mixture with 10 mL blood and 25 IU bovine thrombin; in series 3, the thrombus was removed from the silicone tube after incubation for 60 minutes and incubated in 20 mL of 1% tungsten silicic acid solution for an additional 20 minutes. Radiographic absorption was evaluated by a high-resolution CT scan of the thrombus (Somatom Sensation 16; Siemens Medical Systems, Erlangen, Germany).
In addition to being comparatively expensive, Tantalum showed a high tendency to sedimentation in the syringe and silicone tube and had lower radiographic absorption in the tested concentrations. Incubation with 1% tungsten silicic acid did not produce the significant radiographic absorption necessary for angiography.
All further experiments were therefore performed with a barium sulfate–marked thrombus.
Based on the results of the dilution series with barium sulfate, good mechanical stability, good radiopacity, and low sedimentation were found with 1 g barium sulfate, 25 IU bovine thrombin, and 10 mL autologous blood. Sedimentation of the contrast agent could be minimized by continuous turning of the tube during the first 3 minutes of incubation time.
In general, the method of preparation allowed the generation of thrombi of different shapes and sizes. The chosen setting (number 4) produced homogenous 3-mm thrombi with the following specifications: for each 10 mm of length the thrombus has a volume of approximately 70.9 mm3 (0.71 mL) and a weight of 45 mg containing about 7 mg of barium sulfate.
Angiography
Each animal underwent surgery through a groin approach for preparation of the common femoral artery (CFA) and vein (CFV) under general anesthesia. In animals weighing more than 50 kg, the distance between the CFA and the cervical bifurcation of the common carotid artery (CCA) exceeds the length of most devices. We therefore used pigs weighing 43–47 kg in our study. A 450-mm-long 7F catheter sheath (Arrow; Arrow International, Reading, Pa) was introduced in the CFA and continuously flushed with heparinized physiologic saline (10 U/mL). A central venous catheter was placed in the CFV for constant venous access.
Selective intra-arterial digital subtraction angiography (DSA) was performed on a biplane high-resolution angiography system (Toshiba CAS 500, Tokyo, Japan) with a matrix of 1024 × 1024 pixels. Iopamidol (Iopamiro 300; Bracco, Milan, Italy) was used for vessel contrast. First an angiogram of the carotid vasculature was performed to identify areas suitable for reproducing human intracranial anatomy. Follow-up angiographies during and after thrombus application assessed the precise location of the thrombus after application and the hemodynamic changes. The diameter of the large cranial vessels was measured in lateral and anteroposterior (AP) projection images. The angle of the vessels at the origin from the common and external carotid arteries as well as other vessel characteristics (side branches, kinking) were also evaluated.
Thrombus Application
For selective thromboembolization the preformed thrombus was injected into a 7F guiding catheter (Guider Softip; Boston Scientific Target, Fremont, Calif) positioned in the targeted vessel.
Injection of a thrombus >20 mm led to fragmentation at the entrance of the catheter in most cases. To ensure reproducible and stable thrombus application without fragmentation, we therefore changed the application procedure: a thrombus of the selected size (5–60 mm) was placed into a 20-mL syringe with physiologic saline solution and then injected into a silicone tube (3-mm inner diameter; Clinico Medical) that had also been flushed with saline solution to avoid air embolism. This application step is prone to thrombus fragmentation. Once the thrombus is positioned within the silicone tube, it can be easily inspected and measured. When the silicone tube has been connected to the guide catheter, the thrombus can be applied without further risk of fragmentation. To assess possible vessel damage from the barium sulfate–prepared thrombus, histologic work-up of previously embolized arteries was performed.
Results
Thrombus Properties
In the animal model no adverse effect was seen due to the barium sulfate application during the experiments (5–6 hours). The vital parameters revealed no significant systemic effect on heart rate, arterial blood pressure, or ventilation during this time. Local reaction to the contrast agent such as vasospasm or massive thrombosis was not seen in angiography. Histologic examination of the arteries and the thrombus revealed no intimal damage or pathologic changes of the vessel wall.
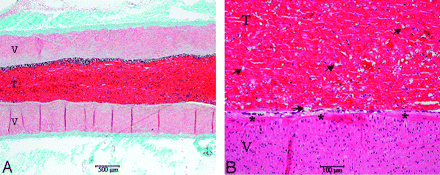
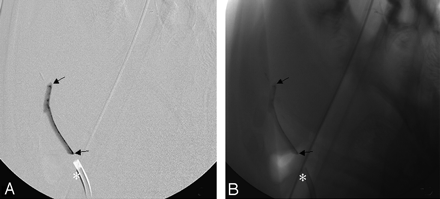
Furthermore, the contrast agent was still embedded in the thrombus 90 minutes after application. No washout of the contrast agent or clot material was seen during the experiments or in the histologic evaluation (Fig 1). Barium sulfate–enhanced thrombus attenuation guaranteed visibility of the thrombus during fluoroscopy and thus facilitated evaluation of hemodynamic features of the thrombus with and without proximal flow arrest. The application and visibility of this thrombus in experiential use are illustrated in Fig 2. The chosen setting allowed stable and selective thromboembolization of the targeted artery, while preinjection of the thrombus into the silicone tube before its attachment to the guiding catheter prevented thrombus fragmentation.
Masson trichrome–stained longitudinal section (A) showing a muscular vessel wall (V) with a thrombus (T) completely occluding the artery 90 minutes after application. A magnified view (B) of the arterial wall and the thrombus in hematoxylin-eosin-stained section revealing evenly distributed barium sulfate particles (arrows) in this whole blot thrombus. The endothelium is intact (asterisk), and no inflammatory changes are observed in the vessel wall.
The barium sulfate–marked thrombus (arrows) during insertion into the lingual artery with (A) and without (B) digital subtraction angiography. The asterisk indicates the location of the 7F guiding catheter.
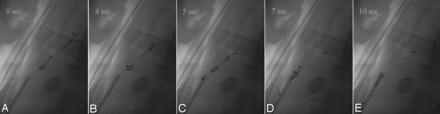
Fragmentation or dislocation of the thrombus due to device maneuvers were easily detected even in unprocessed images (Fig 3). Thrombus movement and especially distal thromboembolization due to thrombus fragmentation were best visualized on DSA images by reason of changes in position versus the previously acquired subtraction mask.
Thrombectomy in the right ICA by using a clot retrieval device (Catch device; Balt, Montmorency, France, upper and lower marker >). The device is unfolded distally to the contrasted thrombus (A), well visualized even in the unprocessed angiogram. The thrombus is partially caught (B) and retrieved by the basket-like device (C). During the pullback maneuver, the thrombus is compressed (D) and subsequently a small fragment (asterisk) remains in the ICA, while a large part of the thrombus is removed (E). The device passes the thrombus at the tip of the 7F guiding catheter and loses this fragment. A small residual fragment is retrieved by the device (E).
Angiographic Results
The vessel size of the model allowed insertion and navigation of standard-sized devices used in humans. Evaluation of vessel characteristics revealed 2 cranial vessels suitable for interventional purposes.
The porcine lingual artery (LA; 2.5–3-mm diameter) originates 30° from the external carotid artery (ECA) in lateral orientations, 60° from the ECA in AP orientations and shows a 80°–90° kinking in the proximal portion of the vessel.
The distal portion has a diameter of 2.5 mm and runs straight for 60 mm with several small side branches. The characteristics of the vessel and small side branches of the LA may be suited as a model for middle cerebral artery (MCA) occlusion in human.
The porcine internal carotid artery (ICA) originates at an angle of 45° from the CCA in lateral orientations and at an angle of 50° in AP orientations. It has a diameter of about 2.5–3 mm, a length of approximately 80–90 mm. It passes through the “rete mirabile,” a network of arterioles whose size (<1 mm) precludes an interventional approach beyond this area.19 The configuration of the vessel, the moderate angle at its origin, and the distal obstacle, comparable to a sharp bifurcation, may let the ICA count as a model for basilar artery (BA) occlusion.
The porcine maxillary artery (MA) originates 20° from the ECA in lateral orientations and 80° in AP orientations. The main branch has a diameter of 6 mm, and the side branches 3–5 mm. The extensive branching inhibited selective thromboembolization and the wide diameter led to far distal obstruction by using a 3-mm thrombus.
Discussion
Mechanical clot retrieval, or thrombectomy, for acute cerebrovascular ischemic stroke is a developing technique of neurointervention. Several studies have reported in vitro results, and a few have described results in a small number of patients by using different clot retrieval devices.10-16,17 These studies revealed the great potential of mechanical thrombectomy and advocated its results to be superior to those of IAT.9,14,15,18
Intra-arterial thrombectomy in general is a well-described procedure for which the first experiences date back to the work of Fogarty in 1967.20 In contrast to its application in peripheral vessels or cardiovascular intervention, thrombectomy in cerebral vessel occlusion is relatively new. Although the mechanical problems associated with the 2 procedures are comparable, the different approach required for brain vessels, as well as the possible fatal consequences of thrombus dislocation or device failure and the lack of fast surgical alternatives, demand new devices and procedures. This need underlines the importance of preclinical testing.
In vitro testing, however, neither provides a comparable hemodynamic situation nor allows for the requisite evaluation of vessel damage such as spasm, dissection, or rupture. A few animal models for thrombus occlusion using a preformed autologous blood thrombus for thromboembolization have been proposed, mostly in small mammals.21-27 These models, however, do not accommodate vascular devices intended for human use, and they do not reproduce human hemodynamics.
Models using larger mammals did not allow selective thromboembolization of targeted vessels.28-30 From our point of view, the best in vivo model for cerebral thromboembolism was described by Ringer et al.31 It permitted similar selective thrombus application and the use of standard-sized devices in a swine model. It did not, however, provide visualization of the thrombus during angiography and therefore provided no reliable information on thrombus fragmentation due to intervention or on thrombus behavior during retrieval.
The animal model described here possesses the characteristics necessary to an ideal model dedicated to evaluating the efficiency and safety of clot retrieval devices in acute stoke. The vessel sizes are comparable to the intracranial setting in humans and allow accurate navigation of the devices. The heart rate of 60–70/min and the arterial blood pressure of 80–120 mm Hg (mean arterial blood pressure, 90–95 mm Hg) provide a comparable hemodynamic situation. The thrombus can be produced to specification, it is reproducible, and its high radiographic absorption allows easy visualization of its interaction with devices and of possible dislocation and fragmentation. Neither the histologic evaluation nor the experimental application revealed any adverse local or systemic reactions to the contrasted thrombus. In this setting a thrombus of any given length between 5 and 80 mm can be produced and injected. Thrombus movement and behavior can be seen in real time, allowing the detection and documentation of the tearing of even small fragments that are immediately dislocated to peripheral vessels.
The selective thromboembolization of targeted vessels with a preformed whole-blood thrombus enables the creation of a standardized setting for repetitive testing. The vessel characteristics of the porcine ICA and the LA reproduce the anatomic setting of an occlusion of the MCA and the BA in human circulation, which are the common localizations for interventional stroke therapy.
This first in vivo model with a visualization is therefore a highly useful basis for the development and evaluation of mechanical thrombectomy in acute cerebrovascular stroke.
Limitations of the introduced animal model concern the thrombus and vessel morphology. Studies on thrombus morphology revealed a variety of different thrombi compositions found in human stroke. Although the predominant thrombus is the whole blood thrombus or “red clot,”32 the compositions (eg, fibrin-rich thrombus or white clot) might have a significant influence on the mechanical stability and therefore the success rate of thrombectomy. The generated whole-blood thrombus advocated in this model might therefore not represent the whole spectrum of mechanical properties of thrombi found in acute stroke. We observed no significant difference in the mechanical stability of the thrombi after the application of barium sulfate in vitro; in vivo, however, the agent might influence the mechanical and chemical properties such as the adhesion force of the thrombus to the vessel wall.
Although the size of the animal and of the selected vessels bear comparison to the intracranial anatomy of humans, the present model does not provide a high grade of kinking or atherosclerotic changes. Unlike humans, therefore, the model exhibits a low tendency to artery dissection. Therefore, it might lead to underestimation of this complication in the evaluation of procedures and devices.
Conclusion
We present a dedicated animal model for mechanical thrombectomy in acute stroke that allows selective and reliable thromboembolization of vessels of sufficient size to test devices intended for human use. It reproduces the anatomic and hemodynamic situation in MCA and BA occlusions and allows visualization of the thrombotic material during angiography and intervention, thus providing unique insights into the behavior of both thrombus and device. These features make our model potentially useful for the development and evaluation of mechanical thrombectomy in acute cerebrovascular stroke.
References
- Received October 6, 2005.
- Accepted after revision October 31, 2005.
- Copyright © American Society of Neuroradiology