Abstract
OBJECTIVE: To describe the CT findings of lymph nodes of the neck involved in peripheral T-cell lymphomas (PTCL).
MATERIALS AND METHODS: Twenty-seven patients with pathologically proved PTCL with involvement of the lymph nodes of the neck were enrolled in this study. We retrospectively evaluated the lymph nodes on CT images with special attention to nodal necrosis, the margin, and enhancement patterns.
RESULTS: In the 27 patients studied, nodal necrosis and ill-defined margin were seen in 11 (41%) and 19 (70%), respectively. Heterogeneous enhancement of enlarged lymph nodes was noted on CT images in 19 (70%) of 27 patients. Homogeneous enhancement without ill-defined margin and/or nodal necrosis was only seen in 6 of 27 patients (22%).
CONCLUSION: Necrosis, an ill-defined margin, and heterogeneous enhancement of enlarged lymph nodes in the neck are relatively common CT features of PTCL. For patients with cervical lymph node enlargement, the presence of these findings may suggest high-grade non-Hodgkin’s lymphoma, including PTCL.
The WHO classification divides lymphoid malignancies into T-cell neoplasms, B-cell neoplasms, and Hodgkin disease. T-cell neoplasms are divided into precursor T-cell neoplasms and peripheral T-cell neoplasms. Of the peripheral T-cell neoplasms, primary extranodal and predominantly nodal diseases are referred to as peripheral T-cell lymphoma (PTCL).1 PTCL comprises several clinicopathologic entities, including nasal-type extranodal natural killer/T-cell lymphoma (NK/T), enteropathy-type T-cell lymphoma, subcutaneous panniculitis-like T-cell lymphoma, mycosis fungoides, anaplastic large T-cell lymphoma, angioimmunoblastic T-cell lymphoma (AITL), hepatosplenic γδ T-cell lymphoma, and peripheral T-cell lymphoma, unspecified (PTCLU).1 PTCL is an uncommon disease entity more prevalent in Asia than in Western countries and accounts for 5% to 30% of all non-Hodgkin lymphomas (NHL).2–4 It has been reported that PTCL has a poorer prognosis than does B-cell lymphoma.5,6
The imaging features for lymph node involvement in lymphoid malignancies have been reported, but most reports have focused on B-cell lymphoma.7–10 To our knowledge, there have been no reports of imaging findings for nodal involvement in PTCL by using helical CT. The purpose of this study, therefore, was to describe the CT findings for nodal involvement in the necks of patients with PTCL.
Materials and Methods
Patients
A computerized search of the medical records from November 1995 to December 2004 at our hospital indicated that 77 patients had PTCL confirmed by excision biopsy and histopathology. We excluded those patients who had received chemotherapy and/or radiation therapy before the CT scan. The interval between the image acquisition and the biopsy for the included patients was ≤30 days (mean, 17.8 days). In this study, we used the minimum axial diameter of the node, with normal nodes not exceeding 11 mm in the jugulodigastric region and 10 mm elsewhere in the head and neck.11 We also evaluated a node as pathologic, irrespective of its nodal size, when it exhibited nodal necrosis. On the basis of imaging criteria for pathologic adenopathy, a determination was made as to whether the neck lymph nodes were involved in the malignant lymphoma. Of the original 77 patients with PTCL, 27 (20 men, 7 women; mean age, 57.5 years) were included in the study. The initial clinical presentations of these patients were a palpable neck mass (n = 20), upper respiratory infection symptoms (n = 3), tonsillar or peritonsillar enlargement (n = 2), an inguinal mass (n = 1), and abdominal pain (n = 1). No patient was infected with human immunodeficiency virus.
Pathologic Confirmation
Histopathologic confirmation of PTCL was obtained from excision biopsies in all patients. The biopsy sites were the cervical lymph node (n = 14), tonsil (n = 6), axillary lymph node (n = 2), inguinal lymph node (n = 2), nasal mucosa (n = 1), tongue (n = 1), and nasopharynx (n = 1). The diagnosis of PTCL was based on a combination of morphologic assessment and immunophenotyping results. The pathologist classified the PTCL lesions according to the WHO classification system.1
Imaging
All CT scans were performed with a multidetector CT (LightSpeed Ultra or LightSpeed 16; GE Medical Systems, Milwaukee, Wis) or a single-detector CT (LightSpeed Qx/I; GE Medical Systems). A total of 90 mL of nonionic contrast medium was administered into an antecubital vein at 3 mL/s by using a power injector. In all patients, scanning started after a delay of 30 seconds from the commencement of injection. Imaging was initiated at the level of the sternal notch and continued toward the skull base with 3.75-mm collimation, 24-second acquisition time, a table speed of 8.75 mm/s, 0.8-second rotation time, 200 mA, and 120 kVp. From the volumetric data, contiguous axial images were reconstructed at 5-mm intervals.
Image Interpretation
The CT images were retrospectively evaluated by consensus of 3 radiologists (J.W.C., S.S.K., E.Y.K.) who were aware that all patients had PTCL. We analyzed all detectable lymph nodes satisfying the criteria for lymph node involvement of malignant lymphoma with special attention to the following features: the presence or absence of necrosis, the margin, and enhancement pattern. We considered an area of low attenuation 10–18 HU) to be evidence of nodal necrosis. The margin and enhancement patterns were categorized as homogeneous or heterogeneous and as well-defined or ill-defined, respectively. When one or more of the involved lymph nodes appeared heterogeneous, the result was reported as heterogeneous. The presence of nodal necrosis and the margin were reported in the same fashion.
Results
The imaging features for all patients are summarized in the (Table. The pathologic diagnoses were PTCLU (n) = 20, nasal-type NK/T (n = 5), and AITL (n = 2). No patients had anaplastic large-cell lymphoma or subcutaneous panniculitis-like T-cell lymphoma. Ten patients (37%) had evidence of both an ill-defined margin and necrosis (Fig 1). Ten patients (37%) showed nodal necrosis (Fig 2) with heterogeneous enhancement (Fig 3). Seventeen patients (63%) showed an ill-defined margin and heterogeneous enhancement. Ill-defined margin alone or heterogeneous enhancement alone were seen in only 1 patient (4%) each. No patients exhibited only nodal necrosis. The classic presentation of malignant lymphoma showing homogeneous enhancement and a well-defined margin without necrosis7 was seen in 6 of 27 patients (22%), of whom 5 had PTCLU and 1 had nasal-type NK/T.
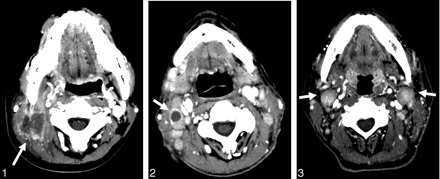
A 21-year-old woman with nasal type natural killer/T-cell lymphoma. Axial CT scans show an irregular-walled necrotic enlarged lymph node with an ill-defined margin in the right level II (arrow).
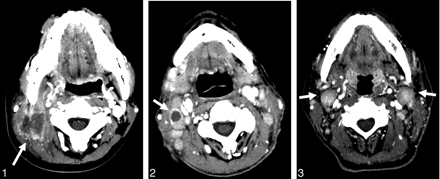
A 72-year-old man with angioimmunoblastic T-cell lymphoma. Axial CT scans show necrotic, smooth-walled lymphadenopathy with a well-defined margin at right level II (arrow).
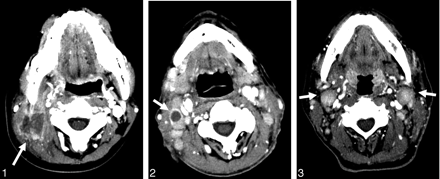
A 55-year-old man with peripheral T-cell lymphoma, unspecified. Axial CT scans reveal heterogeneously enhanced enlarged lymph nodes in the internal jugular chain bilaterally (level II) (arrows). These nodes measure 1.6 cm in minimum axial diameter and have also been palpated abnormally for 6 months and have increased in size.
CT findings in subtypes of peripheral T-cell lymphoma
Discussion
Malignant lymphoma represents the second most common malignancy of the extracranial head and neck region after squamous cell carcinoma and should be considered in the differential diagnosis of any nodal or extranodal masses.9 The classic presentation of lymphoma of the neck with lymph node involvement is enlarged, homogeneous, often bilateral, non-necrotic lymph nodes. The disease almost always spreads into the deep lymphatic chains, particularly the internal jugular chain.7 Harnsberger et al8 reported that central nodal necrosis and extracapsular infiltration, findings often observed in metastatic squamous cell carcinoma of the head and neck, were distinctly less common in lymphomas that had not been treated; they suggested that the presence of these findings should prompt the radiologist to search for an undetected primary squamous neoplasm.
Necrosis is considered to be an uncommon feature of lymphadenopathy in NHL. When this finding is detected in the cervical lymph nodes in the absence of treatment, it could be suggestive of a primary pathology other than NHL, such as metastatic squamous cell carcinoma or tuberculosis of the head and neck. Lee et al7 reported that nodal necrosis was found in 5% of cases of Hodgkin disease and in 13% of cases of NHL, even when the disease was extensive. Harnsberger et al8 found that central necrosis in only one of 12 NHL patients with involvement of the neck lymph nodes. In our study, 11 of 27 patients (41%) showed evidence of necrosis in the involved lymph nodes, which was a higher prevalence than in the previous reports. Therefore, PTCL should remain on the differential diagnosis, even in patients with nodal necrosis. Pathologically, PTCL tumors involve the marginal sinuses of the nodal cortex first, with subsequent involvement of the medulla, blockage of the lymph flow, and resultant medullary necrosis. Thus, the low-attenuation regions seen on the CT images may represent either tumor replacement of normal lymph node tissue or necrosis.12 Furthermore, necrosis is potentially a prognostic indicator in NHL,13 in that necrotic nodes were more prevalent in advanced-stage lymphomas. This observed higher incidence of necrosis in PTCL in this study may therefore be in accordance with the generally poorer prognosis for this disease than for B-cell lymphoma.
Radiologists should be aware that although an area of hypoattenuation is a highly specific sign of metastatic nodal involvement, it may be seen in other malignancies, such as PTCL. Nodal NHL is suggested when large, bilateral nodes are detected in an atypical drainage area8; such findings could be helpful in differentiating NHL from metastatic squamous cell carcinoma. In addition, the benign process of fatty nodal metaplasia, which can be caused by infection or radiation therapy, may mimic the appearance of nodal necrosis.12,14–15 Therefore, attention to the patient’s presentation and history will be helpful in arriving at a correct diagnosis.
On CT images, extracapsular infiltration is suggested by an amorphous, ill-defined margin, irregular nodal capsular enhancement, and infiltration of the surrounding fat.12,16 An ill-defined margin suggesting extracapsular infiltration is considered more indicative of metastatic disease from squamous cell carcinomas than of NHL, if there is no history of previous treatment or recent infection.10 In this study, an ill-defined margin was a common finding, occurring in 19 (70%) of 27 patients. An ill-defined margin suggesting extracapsular infiltration is known to be an important prognostic indicator for squamous cell carcinoma; its presence in metastasized lymph nodes increases the risk of local failure, distant metastases, and decreased survival.17,18 To our knowledge, there have been no reports on the prognostic significance of extracapsular infiltration in NHL, perhaps because it has been considered an uncommon finding in malignant lymphoma. However, the high incidence of an ill-defined margin in this study indicates that it may suggest the possible presence of more aggressive lymphomas, such as PTCL. Moreover, given that extracapsular infiltration can result from infection, surgery, or radiation therapy, it is important to differentiate among these conditions by careful correlation with the patient’s history.12
Nodal involvement in NHL usually appears homogeneous and occasionally displays a variable degree of enhancement.19,20 In previous studies, most high-grade NHL tumors had a more or less inhomogeneous pattern, whereas most low-grade NHLs had a homogeneous appearance; tumors with a severely inhomogeneous pattern were virtually always high-grade tumors.21–23 In our study, 19 patients (70%) manifested heterogeneous enhancement patterns. Although heterogeneous enhancement may be noted in other nodal diseases, such as metastases or tuberculosis, a relatively large proportion of the patients with PTCL in our study showed this enhancement pattern. In general, nodal enhancement seems to imply increased nodal vascularity, and the enhancement patterns of tumors might be influenced by many biologic factors and scanning parameters, such as scanning time. According to the development of CT techniques, more rapid scanning is possible. The higher incidence of heterogeneous enhancement might be partly attributed to shortening of scanning time. Therefore, the value of the heterogeneous enhancement in our patients is limited.
PTCLU accounts for approximately 11% of all NHL in Korea and approximately 4% in Europe.2,24 This category usually manifests as generalized disease; the lymph nodes, liver, and spleen can also be involved. Lee et al25 found that the most common radiologic feature of PTCLU was generalized lymphadenopathy and that, in the disseminated condition, the image features were not distinguishable from those of other subtypes of lymphoma. In our study, lymph nodes affected with PTCLU showed nodal necrosis in 6 of 20 patients (30%), an ill-defined margin in 13 (65%), and heterogeneous enhancement in 13 (65%). These findings were not concordant with the imaging findings from the previous report.25 Again, this may suggest that these radiologic features are indicative of the aggressive clinical course of PTCLU.
Nasal-type NK/T is more common in Asian and South American countries than in Western countries. It accounts for approximately 12% of all NHL in Korea and approximately 2% in Europe.2,24 Nodal involvement in nasal-type NK/T is rare, and the CT findings have not been reported. Our study included 5 patients with nasal-type NK/T, 3 of whom (60%) had nodal necrosis in the involved lymph nodes. Four patients (80%) showed an ill-defined margin and heterogeneous enhancement. Nasal-type NK/T is known to be a very aggressive subtype of PTCL.26,27 From a pathologic standpoint, invasion of the vascular walls in primary nasal-type NK/T tumors by lymphoid cells causes occlusion of the lumen, which is associated with ischemic necrosis of the tumor. Therefore, we suggest that necrosis of lymph nodes involved in nasal-type NK/T might be induced in the same manner as in the main tumor.
AITL is a relatively rare disease, accounting for less than 1% of all NHL.2,24 This disorder is clinically characterized by prominent vascular proliferation; affected lymph nodes show an effaced architecture caused by proliferation of atypical lymphoid cells and arborizing of endothelial venules.28 Patients with AITL present with poor prognostic features.29 In our study, all patients with AITL showed nodal necrosis, an ill-defined margin, and heterogeneous enhancement. Although the number of patients with AITL enrolled in this study was insufficient to establish representative characteristics of nodal involvement, our findings might be related to the poor clinical course and pathologic findings in these patients.
We recognize that this study has some limitations, the first of which is its retrospective nature. Second, the diagnosis of nodal involvement of the lymphomas was based on imaging criteria, though excision biopsies were performed in all patients. Biopsies of the cervical lymph nodes were done in 14 patients, and the remaining patients had biopsies of other nodes or organs. Therefore, we could not directly correlate the enlarged lymph nodes on the CT images with the pathology specimen. Third, our pathologic diagnosis included only 3 pathologic subtypes: PTCLU, nasal-type NK/T, and AITL. Therefore, our study does not present CT findings for all the subtypes of PTCL. Fourth, our study was performed 30 seconds after administering contrast media, which may be part of the cause of the heterogeneous enhancement of the lymph nodes.
In this study, nodal necrosis, an ill-defined margin, and heterogeneous enhancement of enlarged lymph nodes in the neck were relatively more common in PTCL than in other lymphomas. However, head and neck carcinomas, B-cell lymphomas, Hodgkin lymphomas, and granulomatous diseases are more prevalent worldwide than PTCL; therefore, in practice, these findings are still more common in these other diseases.14,15 As such, based on the imaging findings of an involved lymph node, the identification of these disease entities is very difficult. The patient’s history and histologic confirmation are needed to arrive at a correct diagnosis.
In conclusion, necrosis, an ill-defined margin, and heterogeneous enhancement of an enlarged lymph node in the neck are relatively common CT features of PTCL. For patients with cervical lymph node enlargement, the presence of these findings may suggest high-grade non-Hodgkin lymphoma, including PTCL.
Footnotes
This work was supported by a grant (2005) from the Kangwon National University.
References
- Received April 26, 2005.
- Accepted after revision September 25, 2005.
- Copyright © American Society of Neuroradiology