Abstract
Summary: We report a patient with acute hemorrhagic leukoencephalitis with a focus on serial MR imaging findings. Initial MR imaging of a 42-year-old woman revealed a 2.5-cm focal nonhemorrhagic lesion in the left thalamus and internal capsule. Twenty-four days later, fever and altered consciousness developed, and MR imaging showed huge masslike lesions in both frontal lobes, mainly involving the white matter and the genu of the corpus callosum, with massive edematous swelling that contained multifocal small hemorrhages. Most lesions showed high apparent diffusion coefficient value with peripheral small areas of low apparent diffusion coefficient. On follow-up MR imaging obtained 49 days after initial MR imaging, the lesions progressed with increase in extent and development of rim-enhancing necrosis, despite steroid therapy. Following stereotactic biopsy and subsequent high-dose steroid treatment, the patient recovered with some neurologic sequelae. MR imaging obtained at 72 and 126 days revealed residual necrosis and cerebromalacia in the both frontal lobes.
Acute hemorrhagic leukoencephalitis is a rare demyelinating disease characterized by an acute rapidly progressive fulminant inflammation of the white matter, first described by Hurst in 1941 (1, 2). It is regarded as the most severe subform of acute disseminated encephalomyelitis (3). Early diagnosis of acute hemorrhagic leukoencephalitis is critical because it frequently causes severe morbidity within a few days or leads to death, but the patients can survive with early treatment with combinations of corticosteroids, immunoglobulin, cyclophosphamide, and/or plasma exchange (4–6).
To be aware of MR imaging findings of acute hemorrhagic leukoencephalitis together with clinical manifestations and cerebrospinal profile is helpful to the radiologists for the early diagnosis of this entity. To our knowledge, only a few MR imaging findings of pathologically proven acute hemorrhagic leukoencephalitis have been reported in the English literature (7–9). We present the serial MR imaging appearances in a case of acute hemorrhagic leukoencephalitis confirmed by pathology.
Case Report
A 42-year-old woman was admitted to our institution because of fever, alteration of consciousness, and aphasia for 1 day. Twenty-four days before admission, right-sided sensory changes and motor weakness suddenly developed. Initial MR images obtained at that time showed a 2.5-cm nonhemorrhagic focal lesion of T2 high and T1 low intensity in the left thalamus and posterior internal capsule, extending to the left side of the midbrain. She had been treated with prednisolone for the clinical diagnosis of presumed multiple sclerosis or vasculitis, even though the findings of various serologic studies relevant to vasculitis were negative. According to the second MR images obtained 7 days before admission, the lesion decreased in size. Diffusion-weighted imaging and apparent diffusion coefficient map revealed high apparent diffusion coefficient value in most of the lesion with a small focus of low apparent diffusion coefficient at the peripheral portion where the lesion was faintly enhanced on contrast-enhanced T1-weighted imaging.
On admission, the patient presented with increased temperature (39.5°C), free voiding, and drowsy mentality. There was leukocytosis with predominant polymorphonuclear cells in the peripheral blood. Cerebrospinal fluid revealed pleocytosis (white blood cell count of 2100 cells/mm3 with 88% neutrophils and a few red blood cells), increased protein level (176 mg/dL), and decreased glucose level (31 mg/dL, blood glucose of 118 mg/dL). The results of various serologic studies, including polymerase chain reaction and other immunologic and microbiologic studies of the peripheral blood and cerebrospinal fluid, were all were negative.
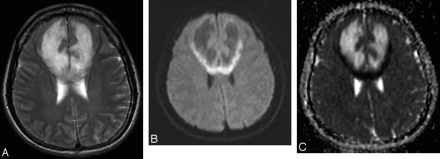
The third MR images obtained at the time of admission showed new large masslike lesions mainly involving the bifrontal white matter and the genu of the corpus callosum with severe ventricle compression (Fig 1). The lesions showed T2 high and T1 low intensity with multiple small foci of dark intensity scattered throughout on the T2-weighted gradient-echo image suggestive of multifocal petechial hemorrhage. The diffusion-weighted images and apparent diffusion coefficient maps revealed increased apparent diffusion coefficients in most of the lesions but decreased apparent diffusion coefficients in the peripheral portions where marginal enhancement was seen on the contrast-enhanced T1-weighted images. No evidence of venous sinus thrombosis was noted on MR venography. With presumed diagnosis of acute fulminant form of multiple sclerosis, acute hemorrhagic leukoencephalitis, or vasculitis, based on clinical and MR imaging findings, medical treatment with corticosteroids was continued.
MR images obtained on admission.
A, Axial T2-weighted fast spin-echo image (TR/TE, 4390/121). B, Diffusion-weighted image (TR, 6500; b value, 1000 seconds/mm2). C, Apparent diffusion coefficient map. T2-weighted image (A) shows new large lesions mainly involving the bifrontal white matter and the genu of the corpus callosum. Diffusion-weighted image (B) and apparent diffusion coefficient map (C) reveal increased apparent diffusion coefficient in most of the lesions, with decreased apparent diffusion coefficient in the peripheral portions.
On the fourth MR images obtained 25 days after admission, the bifrontal lesions had progressed with increase in the extent, and new multifocal areas of rim enhancement with central necrosis appeared in the vicinity.
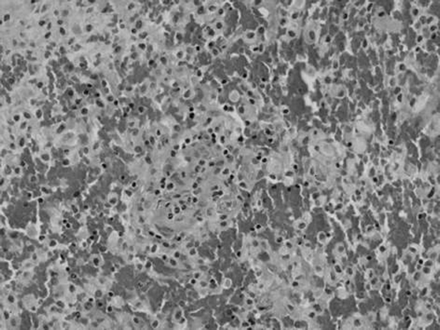
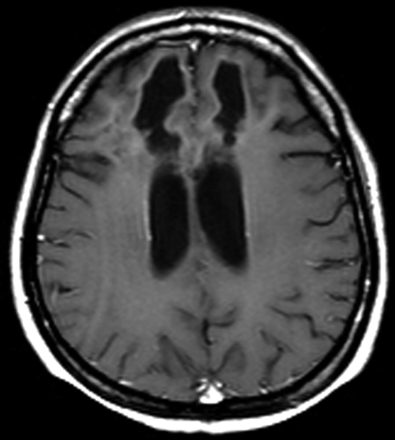
A stereotactic biopsy was performed 1 day (49 days after first symptom onset) after the fourth MR imaging study. A pathologic specimen obtained from the right frontal lobe showed demyelination, diffuse foamy macrophage infiltration, small blood vessel proliferation with perivascular lymphocytic infiltration, and perivascular hemorrhage on sections stained with hematoxylin and eosin, luxol fast blue, Bodian, and CD68 (Fig 2). These findings were consistent with acute hemorrhagic leukoencephalitis with profound demyelination. Afterward, the patient was treated with high-dose corticosteroids, resulting in gradual improvement of the symptoms and signs and eventually complete recovery of consciousness. The fifth and sixth MR images obtained 48 days and 102 days, respectively, after admission showed marked decrease of the mass effect with residual necrosis and cerebromalacia of the bifrontal lesions (Fig 3). Her clinical and neurologic features and cerebrospinal fluid findings have improved without relapse up to now (follow-up period of 4 months).
Histopathologic microscopic finding obtained via stereotactic biopsy of right frontal lobe 26 days after admission. Microscopic photograph shows perivascular mononuclear inflammatory cell cuffing and diffuse infiltration of macrophages, with perivascular hemorrhages, which separate infiltration of macrophages (hematoxylin and eosin, original magnification ×200).
MR image obtained 102 days after admission. Contrast-enhanced axial T1-weighted spin-echo image (TR/TE, 650/17) shows residual cystic necrosis and cerebromalacia of the both frontal lobes.
Discussion
In our case, differential diagnosis based on MR imaging findings includes fulminant multiple sclerosis, acute disseminated encephalomyelitis, acute hemorrhagic leukoencephalitis, infectious encephalitis, vasculitis, and venous sinus thrombosis. Infectious encephalitis including herpes simplex encephalitis, and other viral, bacterial, or fungal infections could be excluded with MR imaging findings of nonsuppurative nonenhancing diffuse edematous lesions, mainly involving the white matter of the cerebral hemispheres together with negative serologic and microbial examinations of cerebrospinal fluid. Vasculitis may reveal the acute clinical course and mimic demyelinating disease but usually appears as smaller multiple lesions and involves the cortices as well as the white matter, especially when the lesions are extensive as in our case. Various serologic tests for collagen diseases may also be helpful in the diagnosis of vasculitis, but their findings were all negative in our case. Venous sinus thrombosis could be excluded with MR venography. It may be difficult to differentiate fulminant multiple sclerosis, acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis.
Multiple sclerosis is usually a polyphasic chronic disease but rarely may have an acute fulminant course. Even in acute fulminant multiple sclerosis, however, high fever, marked leukocytosis in the blood, and marked pleocytosis with predominant neutrophils in the cerebrospinal fluid, as seen in our case, are not usually present. In addition, hemorrhagic foci on MR imaging is usually not seen in multiple sclerosis (10).
Acute disseminated encephalomyelitis is a postinfectious, immune-mediated demyelinating disease that develops subsequent to a viral infection or vaccination against viral diseases and usually has an acute monophasic clinical course. Acute hemorrhagic leukoencephalitis is the most fulminant form of demyelinating disease affecting young adults. Acute disseminated encephalomyelitis and acute hemorrhagic leukoencephalitis share many features and may be part of a spectrum of disease with the same fundamental process resulting from an autoimmune process, rather than distinct entities. Acute hemorrhagic leukoencephalitis is considered to be a hyperacute form of the more common acute disseminated encephalomyelitis (3, 7, 11, 12). Tenembaum et al (3) classified acute hemorrhagic leukoencephalitis as the most severe subform of acute disseminated encephalomyelitis. The clinical course of acute hemorrhagic leukoencephalitis is more fulminant than that of the usual acute disseminated encephalomyelitis and frequently is associated with the cerebrospinal fluid profile of pleocytosis with predominant polymorphonuclear cells, in contrast to lymphocytic predominance in acute disseminated encephalomyelitis (11). On MR imaging, the lesions of acute hemorrhagic leukoencephalitis tend to be larger and associated with more edema and mass effect than those in acute disseminated encephalomyelitis (5, 7) and frequently show foci of hemorrhage, unlike acute disseminated encephalomyelitis (11). On pathology, acute hemorrhagic leukoencephalitis produces a predominantly neutrophilic infiltrate with perivascular hemorrhage and necrotizing vasculitis, whereas in acute disseminated encephalomyelitis, perivascular infiltrates consist predominantly of lymphocytes and macrophages with relatively few neutrophils, no perivascular hemorrhage, and vascular necrosis (7, 11).
In our case, the combination of acute clinical course, cerebrospinal fluid profiles, MR imaging findings, and pathologic findings strongly support the diagnosis of acute hemorrhagic leukoencephalitis, even though overt symptoms of a preceding infection could not be found and despite apparent biphasic clinical and MR imaging features in an interval of less than 1 month. Tenembaum et al (3) suggested the term “biphasic disseminated encephalomyelitis” in 10% of their 84 patients with acute disseminated encephalomyelitis, including acute hemorrhagic leukoencephalitis in a long-term follow-up study. Our case may be a biphasic form of acute hemorrhagic leukoencephalitis but should be followed longer.
Previous CT or MR imaging descriptions of acute hemorrhagic leukoencephalitis include asymmetric unilateral or bilateral white matter abnormalities of the cerebral hemispheres, particularly the frontal and parietal lobes, which extend from the periventricular region to the gray–white junction with relative sparing of the cortex. The basal ganglia, thalamus, brain stem, cerebellum, and spinal cord may also be affected (1, 5, 7–9, 11, 12). The signal intensity abnormalities of the lesions are likely due to increased water content associated with edema and inflammation. In later stages, the signal intensity changes are more likely to represent demyelination and axonal loss (7). Contrast enhancement of the lesions is variable in cases, depending on the different age and severity in degree of the lesions because the breakdown of the blood–brain barrier in the acute inflammatory phase is the pathologic correlate of this appearance (13). The report of Mader et al (12) showed diffusion-weighted imaging findings in acute hemorrhagic leukoencephalitis. In contrast to our case showing low diffusivity only in the peripheral portions of the lesions, their case revealed low diffusivity in most areas of the bilateral thalami and right basal ganglia involved. It is suggested that this diffusion abnormality may be caused by cytotoxic edema secondary to the toxic effect of acute inflammation or acute vasculitis with subsequent vessel occlusion, which might also explain vessel wall necrosis causing hemorrhage.
In our case, high-dose steroid therapy led to the improved clinical outcome, even though some clinical sequelae remained, and the last 2 follow-up MR imaging studies showed residual necrosis with cerebromalacia in the bifrontal regions. Reports of favorable neurologic outcomes with aggressive treatment highlight the importance of early recognition of acute hemorrhagic leukoencephalitis, which would be aided by early MR imaging (4–6).
References
- Received August 17, 2004.
- Accepted after revision November 8, 2004.
- Copyright © American Society of Neuroradiology