Abstract
BACKGROUND AND PURPOSE: Direct cortical stimulation studies suggest that responsive naming is more widely distributed within the temporal lobe than confrontation naming and involves anterior temporal regions typically resected in a standard temporal lobectomy. The aim of the current study was to further demonstrate the anatomic dissociation between confrontation and responsive naming by using functional MR imaging (fMRI).
METHODS: Twenty participants underwent fMRI while performing either a confrontation or responsive naming task. Regions of interest were identified within the anterior and posterior temporal lobe.
RESULTS: Responsive naming produced more activation than confrontation naming within the dominant temporal lobe, with activation extending into the temporal pole. Activation in the dominant temporal lobe associated with responsive naming was observed in the superior, middle, and inferior temporal gyri but was limited to the middle temporal gyrus for confrontation naming. Although both naming tasks produced activation within the posterior temporal region of interest in all participants, responsive and confrontation naming produced activation within the anterior temporal region of interest in 90% versus 60% of the sample, respectively. Areas of the dominant hemisphere activated by both tasks included parts of the middle occipital and middle temporal gyri, inferior frontal lobe, and hippocampus, among others.
CONCLUSION: Findings are consistent with cortical stimulation studies and suggest that responsive naming produces more widespread activation within the temporal lobe compared with confrontation naming. The activation more often included anterior temporal regions during responsive naming as compared with confrontation naming. In clinical cases where the functional assessment of the temporal lobe—particularly the anterior regions—is important, the current results suggest responsive naming should be a useful fMRI paradigm and may ultimately help predict the risk of postsurgical language changes.
Word finding is typically measured by using confrontation naming in which a person is required to generate the name of visually presented pictures of objects. An alternate approach requires individuals to generate an object name in response to a verbal definition; this type of task has been referred to as auditory responsive naming or just responsive naming. There is evidence that responsive naming is more sensitive to the word-finding problems associated with temporal lobe epilepsy (TLE) of the dominant hemisphere than confrontation naming (1).
Cortical stimulation studies in patients with epilepsy have suggested that there is some anatomic dissociation between areas in the dominant temporal lobe involved in confrontation and responsive naming (2, 3). Both Malow et al (2) and Hamberger et al (3) found that stimulation of anterior lateral temporal cortex disrupted responsive naming, whereas confrontation naming was rarely disrupted by stimulation of areas in this region. In contrast, stimulation of sites in the posterior region of the dominant temporal lobe most often disrupted both responsive and confrontation naming. Such findings support a degree of anatomic dissociation between responsive and confrontation naming that may reflect modality-specific processing.
Individuals with a left hemisphere epileptic focus, particularly those with a history of an early brain insult, however, are more likely to have abnormal language organization (4, 5). Thus, inferring typical brain organization from patients who are known to be at risk for abnormal organization is problematic.
Functional MR imaging (fMRI) is another approach that has been used to map functional brain topography with high spatial and temporal resolution (6–12). It uses blood oxygen level–dependent signal intensity changes to map cortical areas, which are activated during a specific task compared with a baseline task (13). It has the advantage of being a completely noninvasive technique that can be used with healthy controls. In recent years, fMRI-based assessment of language laterality and localization has been increasingly used in patients who will be undergoing resection of a cortical lesion or seizure focus (14).
Adequate functional assessment of the dominant temporal lobe (including anterior regions) is important when it is the possible target of a surgical resection. This is the case in a standard temporal lobectomy, in which the anterior two thirds of the temporal lobe, including a large area of lateral cortex, is resected (15, 16). Thus, the identification of tasks that are both disrupted by direct cortical stimulation of anterior temporal cortex and associated with fMRI activation of this region in healthy individuals will likely have important clinical applications in terms of surgical planning and predicting the risk for postsurgical language changes.
The aim of this study was to attempt to replicate the cortical mapping studies comparing confrontation naming and responsive naming in healthy individuals by using fMRI. On the basis of previous studies, it was hypothesized that responsive naming would produce temporal activation to a greater extent and would more often include activation of anterior temporal regions than confrontation naming.
Methods
Participants.
Participants in this study included 20 healthy right-handers. They were recruited from a variety of sources, including undergraduate college courses and through word of mouth at a medical center. The study was approved by the local institutional review board, and all participants gave appropriate consent. Ten subjects completed the responsive naming task and 10 completed the confrontation naming task. Because confrontation naming was part of a larger fMRI study protocol and responsive naming was added later in the project, only 2 of the subjects completed both naming tasks. The individual data for these 2 subjects are presented separately in the Results section. Within the sample of 10 subjects who completed the responsive naming task, the mean age of the sample was 36.2 years (SD = 7.6 years), with a range of 28–49 years. Within the sample that completed the confrontation naming task, the average age was 39.5 years (SD = 9.3 years), with a range of 28–52 years. The groups were matched for sex distribution; in both groups, 80% of the subjects were women.
Imaging Tasks.
All tasks were block-design paradigms with active blocks of varying durations lasting 12–30 seconds alternating with baseline blocks of similar durations. The total time for each paradigm was 6 minutes 32 seconds. The visual stimuli were projected through an LCD projector (XG-G20XU; Sharp Electronics, Mahwah, NJ) outside the scanning room to a screen located at the end of the scanner bed by using Presentation software (www.neurobs.com). The subject viewed the screen via a mirror on top of the head coil, and special MR imaging–compatible headphones (Resonance Technologies, Northridge, CA) were used to transmit the auditory stimuli. The subject’s head was restrained with a moldable air bag (Vac-Fix-Bionix, Toledo, OH) to help reduce head motion.
For the confrontation naming task, subjects viewed line drawings from the Boston Naming Test (17) every 3 seconds and were instructed to name covertly the object pictured. The baseline condition consisted of the presentation of sets of vertical, horizontal, diagonal, and crossing lines to control for low-level visual perception. Subjects were instructed to attend closely to these images but not to respond in any way. For the experimental condition of the responsive naming task, subjects heard short definitions of various nouns and had to name the object covertly. Items from Hamberger and Seidel’s Auditory Naming Test were used (18). For example, subjects heard a short definition, such as “an instrument you beat with sticks,” and had to generate the word “drum” covertly. The baseline condition consisted of short phrases of the same duration as the definitions presented in the experimental condition but played backward, to control for low-level auditory perception. Behavioral data were collected outside the scanner by using an alternate form of the responsive naming tasks. All subjects achieved a high degree of accuracy (correctly naming approximately 97% of the items). These results are similar to published norms for this task (18). Behavioral data were not collected for the confrontation naming task. The stimuli, however, were taken from the Boston Naming Tests; published normative data for this test for subjects of similar age (by using the group mean age) indicate that 93% of all stimuli are typically named correctly (19).
Imaging.
For the functional images, 21 contiguous 5-mm axial sections were acquired with a gradient echo, echo-planar imaging sequence (TR, 2 seconds; TE, 50 msec; flip angle, 90°; field of view [FOV], 22 cm; 64 × 64 matrix) by using a 1.5T GE Signa NV/I MR imaging system (GE Medical Systems, Milwaukee, WI). Each functional acquisition run contained 196 image volumes, and the first 4 image volumes were removed. A 3D T1-weighted image was acquired by using a fast SGPR sequence (TR, 8.7 msec; TE, 1.8 msec; flip angle, 15°; FOV, 22 cm; 256 × 256 matrix; section thickness, 1.2 mm; bandwidth, 15.63 kHz) for anatomic reference.
Analysis.
The echo-planar images were reconstructed by using standard Fourier transformation combined with image-phase correction to reduce the N/2 ghost artifact (20). The images were then motion corrected with a 3D registration algorithm (21), and the statistical analysis was performed with analysis of functional neuroimages (22).
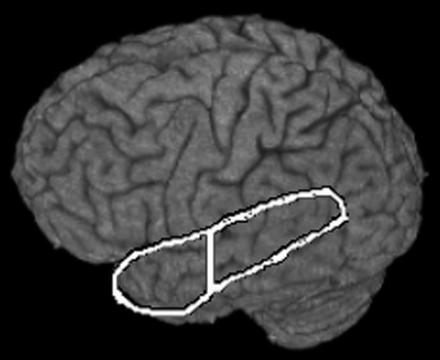
Statistical maps were generated through a multiple regression algorithm by using a boxcar (6-second lag) reference waveform with linear trends included as covariates. Activation maps were created by using a combination of a P value threshold (P < .001) generated from the regression statistics and a minimum cluster size (23, 24) to achieve a significance level <.05 by using AlphaSim (22). The statistical maps were transformed to Talairach coordinates by using a transformation derived from the 3D anatomic dataset (25). Regions of interest were hand drawn according to Talairach coordinates for the anterior and posterior temporal lobe of the left hemisphere (26). Figure 1 depicts the anterior and posterior regions of interest. The anterior temporal region of interest was a small region of interest covering the entire lobe anterior to the Talairach coordinate y = −20 and the posterior temporal region of interest covered the area in the temporal lobe posterior to y = −20. The posterior border of the anterior temporal region of interest at the most superior location is 5 cm from the anterior pole, and the posterior border at a point midway from the inferior and superior borders is approximately 4.1 cm from the anterior pole (15, 16) (this is approximately the cutoff used in Hamberger et al [3] and roughly corresponds to the area of temporal cortex resected in a standard temporal lobectomy). Volumes of activation within each region of interest (left and right) were calculated by counting active voxels within the region of interest.
Temporal regions of interest.
Vertical line represents Talairach coordinate y = −20, which delineates the anterior region of interest from the posterior region of interest.
For the group analysis the statistical maps were smoothed (without threshold) with a 6-mm full width half maximum Gaussian kernel to compensate for residual differences after normalization. Group averages for each task were performed by calculating the mean of the regression coefficients for each voxel and the corresponding t statistic of the mean. Functional maps were created by applying a threshold of P < .01. In addition, a voxel-wise t test was used to compare the differences between the group means for each task.
Results
Group Analysis.
Table 1 presents the Talairach coordinates for activation associated with each naming task within the temporal lobe of the left hemisphere. Responsive naming, compared with baseline, was associated with activation of the middle, inferior, and superior temporal gyri. Other areas of activation within the dominant hemisphere (data not shown) included extensive activation throughout the inferior frontal gyrus and insula. There was also activation in the superior frontal, middle frontal, precentral, inferior parietal, parahippocampal, and fusiform gyri (Brodmann area [BA] 37), as well as the basal ganglia and thalamus. The parahippocampal activation extended into the hippocampus. Activation occurred in the right hemisphere within the inferior frontal, superior temporal, cingulate, and parahippocampal gyri, as well as the insula, basal ganglia, and cerebellum. There was also a large activation cluster extending from the superior frontal gyrus through the medial frontal and cingulate gyri that covered both the left and right hemispheres.
Group results: left hemisphere temporal lobe
Temporal activation for confrontation naming compared with baseline was limited to the middle temporal gyrus. Extratemporal activation included inferior frontal and parahippocampal gyri of the dominant hemisphere. The parahippocampal activation in the left hemisphere extended into the hippocampus. There was also activation of the lingual, cuneus, middle occipital, fusiform (BA 37), precentral, anterior cingulated, and inferior parietal gyri, as well as within the insula, basal ganglia, and thalamus of the left hemisphere. Activation occurred in the right hemisphere within the inferior frontal, precentral, precuneus, middle occipital, inferior parietal, cingulate, parahippocampal, and fusiform gyri (BA 37), as well as the insula, thalamus, and basal ganglia. Similar to responsive naming there was a large cluster of activation extending from the superior frontal gyrus through the medial frontal and cingulate gyri covering both the left and right hemispheres.
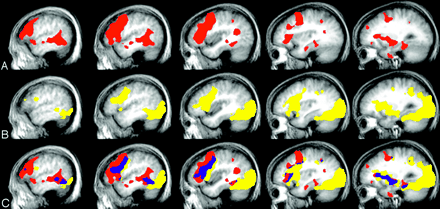
Figure 2 shows the group activation maps for both language tasks. Responsive naming produced more activation compared with the confrontation naming task within the temporal lobe, with the largest activation cluster extending from the posterior superior temporal gyrus through the middle temporal gyrus to anterior and inferior portions of the temporal lobe. Table 1 also includes the Talairach coordinates that are associated with significant differences between the group means for responsive versus confrontation naming within the temporal lobe. The responsive naming versus confrontation naming contrast resulted in significant activation within the inferior, middle, and superior temporal gyri of the dominant hemisphere. Other areas outside of the temporal lobe were also identified in this contrast, most notably in the inferior frontal lobe (BA 9/46; data not shown). The confrontation naming versus responsive naming contrast indicated that confrontation naming was not associated with increased activation anywhere in the temporal lobe as compared with responsive naming. The confrontation versus responsive naming contrast did result in significant activation in several extratemporal sites, including the cuneus and middle occipital gyri, as well as some other areas, including the insula, cingulate, parahippocampal, and inferior parietal gyri (data not shown). In general, the confrontation naming task was also associated with greater activation of the right hemisphere compared with the responsive naming task.
Group activation, left hemisphere.
A, Responsive naming; B, confrontation naming; C, overlap: yellow, confrontation naming; red, responsive naming; blue, overlap.
There were several areas of activation that were associated with both naming tasks. Figure 2 (row C) also depicts the overlapping activation for the group analyses across the 2 naming tasks. In the dominant hemisphere, task activation overlap included areas in the middle occipital and middle temporal gyri, the inferior frontal and precentral gyri, the medial frontal, middle frontal and cingulate gyri, the hippocampus and parahippocampal gyrus, the inferior parietal gyrus, and the basal ganglia. Areas of overlap in the right hemisphere were largest in the inferior frontal gyrus, parahippocampal gyrus, basal ganglia, insula, and cerebellum.
Individual Analysis.
To further investigate differences in activation patterns between the 2 naming tasks at an individual subject level, the number of participants producing activation within each region of interest and the volume of activation in each region of interest were examined. Responsive naming was associated with activation in 90% of the participants within the anterior temporal region of interest and 100% of participants within the posterior temporal region of interest. Confrontation naming also produced activation within 100% of the subjects in the posterior temporal region of interest, but only 60% of subjects in the anterior temporal region of interest.
Table 2 includes the average volume of activation within the regions of interest for each task. Analysis of variance was used to examine differences in activation for each task across the 2 regions of interest. There was a significant effect for both the region of interest (P = 3.0 × 10−6) and the task (P = .01). The main effect for task indicates that, overall, responsive naming produced more activation within the temporal lobe than the confrontation naming task. The main effect for region of interest indicates that, regardless of task, there was more activation within the posterior temporal region of interest than in the anterior temporal region of interest. There was only a nonsignificant trend for an interaction between task and region of interest (P = .15), which provides just weak evidence for a possible difference in activation between anterior and posterior regions for confrontation naming compared with responsive naming (with confrontation naming showing somewhat greater of a difference in activation between the 2 regions of interest).
Individual results
Comparison of Activation in Subjects Who Completed Both Tasks.
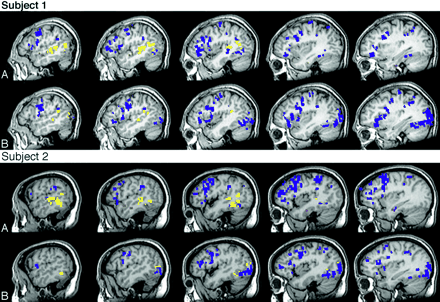
Table 2 includes the activation volume for each individual subject under the naming conditions in the anterior and posterior temporal regions of interest, including the 2 subjects who completed both tasks. The comparison of responsive naming to confrontation naming for the 2 individual subjects who participated in both experiments were similar to the comparison of the tasks for the nonoverlapping subjects; there was more activation detected within both regions of interest for responsive naming compared with confrontation naming. There was minimal activation within the anterior temporal region of interest for both subjects during confrontation naming. In fact, within the anterior temporal region of interest, activation associated with confrontation naming was not detected at all for Subject 2 and the activation detected for Subject 1 was on the outside borders of the anterior temporal region of interest. Figure 3 shows the activation patterns for each task for these 2 subjects. Both subjects produced activation within the anterior temporal region of interest for responsive naming that was near regions detected in the group analyses.
Individual activation, left hemisphere.
Activation patterns for the 2 subjects who participated in each experiment for (A) responsive naming and (B) confrontation naming. Blue, activation external to the temporal regions of interest; yellow, activation within the posterior temporal region of interest; and red, activation within the anterior temporal region of interest.
Discussion
The results of this study suggest that there are some anatomically distinct sites of activation for responsive naming and confrontation naming. Group activation associated with the responsive naming task produced more widespread activation of the dominant temporal lobe, particularly within the superior and middle temporal gyri, which extended into anterior portions of the temporal lobe. This degree of mid- to anterior temporal activation was not observed in association with the confrontation naming task. Even at the individual subject level, activation associated with confrontation naming was not as consistently produced in the anterior temporal region of interest as it was with responsive naming (60% vs 90% of the samples showed activation, respectively). As such, our findings by using fMRI are quite similar to the direct cortical stimulation studies, which showed that auditory responsive naming, but not confrontation naming, was disrupted during stimulation of locations within the anterior portions of the temporal lobe (extending about 4–5 cm from the temporal pole).
The results of the fMRI activation for each task within the posterior temporal region of interest, in comparison to the previous direct cortical stimulation studies, are not as straightforward. The 2 previous stimulation studies reported somewhat different findings with respect to the pattern of disruption associated with the 2 naming tasks within the posterior temporal lobe. Malow et al (2) reported that responsive naming was more disrupted than confrontation naming in the posterior part of the superior and middle temporal gyri, whereas there was a fairly equal degree of disruption of function across the 2 naming tasks in the posterior part of the inferior temporal gyrus. Hamberger et al (3) reported that stimulation within the posterior temporal lobe most often disrupted both confrontation and responsive naming. Our findings revealed some degree of activation within the posterior temporal region of interest during both tasks in all subjects. Responsive naming, however, produced more overall activation in both temporal regions of interest, including the posterior region of interest.
Unfortunately, we were limited to having only 2 subjects who completed both the responsive and confrontation naming tasks. The individual results of the 2 subjects who completed both naming tasks parallel the group results in showing more activation detected in both regions of interest during responsive naming, with confrontation naming producing little if any activation of the anterior temporal region of interest. There have now been a number of studies examining fMRI-related activation associated with various versions of confrontation naming paradigms. Results of the current study are consistent with previous studies showing activation associated with confrontation naming most often involves temporal-occipital cortices (BAs 37, 19, and 18) and the inferior frontal gyrus (7, 27). Most studies have used covert responses during confrontation naming, as done in the current study. Studies that have used overt responses within the scanner, however, have also produced highly similar results (28).
We are aware of only a single study that has examined fMRI-related activation associated with a responsive naming task. Similar to the current study, Balsamo et al (29) reported strong activation within the superior and middle temporal gyri associated with their responsive naming task, with the group analysis appearing to show activation extending into the superior aspects of the temporal lobe. The Balsamo et al study, however, involved young children (mean age, 8.5 years) whose language and semantic network organization may be quite different from those of adults. Furthermore, these authors used a “rest” baseline, which can be problematic (30, 31). Finally, the previous study did not include a confrontation naming task, so activation associated with responsive and confrontation naming could not be compared.
Previous studies have shown that the hippocampus of the speech-dominant hemisphere is a significant component of the neuroanatomic network involved in naming (32–34). In line with such research, the current study also showed that both naming tasks produced hippocampal and parahippocampal activation.
The dissociation between responsive and confrontation naming may reflect modality-specific subsystems involved in word retrieval. Despite the use of an auditory baseline to control for low-level auditory processing, the responsive naming task produced activation throughout much of the temporal lobe (including, but not limited to, primary and secondary auditory cortex). Such activation was strongly lateralized to the left, which suggests that auditory processing was specific to the lexical nature of the stimuli. Activation of inferior frontal regions by both tasks is consistent with other fMRI studies that have compared areas of common activation across different language tasks and suggests that, though word selection and retrieval may be somewhat modality specific (11, 35), other aspects of language such as articulatory planning activate similar areas of the inferior frontal lobe. The finding that both tasks activated a posterior region within the fusiform gyrus is also consistent with previous research that has suggested that BAs 37 and 20 are important in word selection and are believed to be multimodal (28,36–39), receiving input from auditory, visual, and somatosensory cortices (10, 40–43).
Unlike the confrontation naming task, responsive naming, in addition to word retrieval, requires sentence comprehension. A number of neuroimaging studies have now shown that sentence comprehension involves a distributed frontal and temporoparietal neural network—particularly within the dominant hemisphere—including the temporal pole (44, 45). The anterior temporal lobe may be particularly sensitive to both the semantic and the syntactic demands of the task and may help account for the increased anterior temporal activation during responsive naming as compared with during confrontation naming (44, 46).
Both Hamberger and Tamny (47) and Bell et al (1) have found that responsive naming is more sensitive than confrontation naming to the word retrieval deficits associated with TLE of the dominant hemisphere. Positron-emission tomography (PET) studies have shown that, even in epilepsy patients with hippocampal sclerosis, hypometabolism is not restricted to mesial temporal structures, but generally extends into most of the temporal lobe (48–50). It is this lateral temporal lobe involvement that, in addition to the hippocampal dysfunction, likely produces the mild naming deficits often associated with dominant TLE. The larger area of temporal lobe recruitment during responsive naming observed both in this fMRI study and in the direct cortical stimulation studies may account for the increased sensitivity of this task (compared with confrontation naming) in detecting the language deficits within the TLE population.
There are some limitations to this study. First, all behavioral responses in the scanner were made covertly, so it is not possible to confirm that the subjects were performing the task correctly. However, participants received extensive training before scanning and were administered an alternate form of the responsive naming task after scanning. These results showed that participants were highly accurate in their responses (accurately named approximately 97% of the items on an alternate form of the task). We do not have behavioral data on the confrontation naming test that would enable a direct comparison of performance levels, but normative studies also indicate that healthy controls of similar age to our sample name approximately 94% of the items on the Boston Naming Test (19). Use of naming tasks strictly matched on difficulty level should be used in future studies.
Group activation associated with responsive naming was minimal in the inferior regions of the temporal pole. This appears to be at odds with the results of Hamberger et al, who reported disruption of this task at multiple sites in this region (3). Limited activation of this area may be related to the MR imaging signal intensity loss due to magnetic susceptibility artifacts from the nearby auditory canal, mastoid air cells, and petrous bone (51). In fact, a study by Devlin et al (52) compared PET activation with fMRI activation for a semantic task and found temporal pole activation with PET but not fMRI. Those researchers also found that analysis of the echo-planar images showed 82% of the voxels in the temporal pole had a signal intensity loss >25%. The use of shimming or acquiring extra images are approaches that may be used to compensate for, or reduce these artifacts (53, 54) and should be the focus of future research. Another limitation to our study findings, which could affect clinical applicability, was that responsive naming, though producing activation in the anterior temporal region of interest in 90% of our participants, fell short of producing activation in the entire sample. Ideally, fMRI tasks that are going to be used to map functions in clinical samples should produces activation within a given cortical region in all healthy controls. Anterior temporal regions are generally associated with low fMRI signal intensity to noise. Increased detection of activation would likely be achieved by increasing the degrees of freedom associated with the statistical tests. This can be done by repeating the same tasks in 2 different fMRI acquisitions and then combining the tasks or increasing the time points within the fMRI acquisition. Further investigation of the activation associated with responsive naming in a larger sample would help elucidate what percent of healthy controls produce activation in the anterior temporal lobe during this task.
In summary, results of this study support the 2 previous cortical stimulation studies suggesting that responsive naming is, in part, subserved by areas of the temporal cortex that are anterior to those areas associated with confrontation naming (2, 3). Adequate assessment of anterior temporal lobe functions through the identification of tasks such as responsive naming is critical to surgical planning, because they are likely to be useful in predicting the risk of the word-finding changes that can occur after a temporal lobectomy of the dominant hemisphere. Many of the fMRI language paradigms currently in use do not produce activation within the anterior temporal lobe. The selection of language paradigms must be chosen carefully and will vary as a function of the location of the planned surgical resection. We hypothesize that fMRI activation patterns within the anterior temporal lobe associated with responsive naming or other tasks that activate this region may be better predictors of post–temporal lobectomy language outcome than other fMRI paradigms that do not produce activation in this region in healthy individuals. In support of this prediction, the results of Sabsevitz et al (55) suggest that language-related fMRI activation of a temporal region of interest better predicted postsurgical language changes than activation in an inferior frontal region of interest. Future studies examining patterns of temporal lobe fMRI activation associated with responsive naming and other language tasks and detailed postoperative neuropsychological assessment will be useful in determining if they yield differential information in predicting language outcome after temporal lobectomy.
Footnotes
Presented in part as a poster at the 58th annual meeting of the American Epilepsy Society, December 3–8, 2004, New Orleans, LA.
References
- Received February 22, 2005.
- Accepted after revision May 13, 2005.
- Copyright © American Society of Neuroradiology