Abstract
BACKGROUND AND PURPOSE: The management of thrombus formation during coil placement in an intracranial aneurysm is important in minimizing periprocedural morbidity and mortality. We report on seven cases in which the primary treatment for thrombus formation during such coil placement was intra-arterial abciximab infusion.
METHODS: Clinical and radiologic records of 100 consecutive patients who underwent coil placement in intracranial aneurysms at our institution during a 1-year period were reviewed. We identified seven cases (four ruptured aneurysms, three unruptured aneurysms) in which thrombus formation occurred during the procedure.
RESULTS: Intra-arterial abciximab infusion, up to 5 mg, completely dissolved the thrombus in four of seven patients and almost completely dissolved it in two. In one patient with distal emboli, recanalization was not achieved. In two patients, an intravenous bolus of abciximab without 12-hour infusion was also given adjunctively. In one patient, leakage of contrast material occurred; this was related to the intra-arterial infusion. Clinically, no new neurologic deficits were directly related to the intra-arterial abciximab infusion. Six patients had good clinical outcome, and one patient died.
CONCLUSION: Relatively low-dose, intra-arterial abciximab infusion can immediately dissolve an acute thrombus that forms during intracranial aneurysm coil placement. Although neither the optimal dose of intra-arterial abciximab nor the need to supplement the intra-arterial infusion with intravenous administration was established, we preliminarily found that low-dose intra-arterial abciximab infusion may be relatively effective and safe in this setting, even in patients with acute subarachnoid hemorrhage.
Thromboembolism is the most common source of periprocedural morbidity associated with the treatment of intracranial aneurysms with detachable coils. The estimated incidence of thromboembolism is in the range of 3–10%, with permanent deficits estimated to occur in 3–5% of patients (1–5). Among the causes of thromboembolism, thrombus formation at the coil–parent artery interface is a potential major complication and poses a treatment dilemma, particularly in the setting of a ruptured aneurysm (6). The present endovascular management of acute thrombus formation during intracranial aneurysm coil placement includes medical treatment with hypervolemia; increased intravenous heparin; periprocedural and postprocedural administration of antiplatelet agents; intra-arterial thrombolysis with urokinase or tissue plasminogen activator (t-PA); and, more recently, intravenous bolus administration and infusion of potent glycoprotein IIB-IIIA inhibitors such as ReoPro (abciximab; Eli Lilly, Indianapolis, IN) (6–10).
Better regimens are needed to treat this potentially devastating complication. The use of thrombolytics such as urokinase and t-PA in patients with aneurysmal subarachnoid hemorrhage is controversial, particularly given the documented risk of fatal intracranial rehemorrhage (8). Case reports have described the use of an intravenous bolus and infusion of glycoprotein IIB-IIIA inhibitor abciximab as salvage therapy for thrombus formation during intracranial coil placement in unruptured aneurysms (7, 9, 10). However, intravenous doses of abciximab, including the recommended 12-hour infusion with its prolonged half-life, substantially increases the risk of the bleeding complications, both intracranially and systemically; this approach has not been advocated for ruptured aneurysms (6, 11–13). In particular, if pre-existing infarcted areas are present, they may further increase the risk of intracranial bleeding.
We report a series of seven cases (four ruptured aneurysms, three unruptured) in which thrombus formed during intracranial aneurysm coil placement. In each case, the primary treatment was low-dose intra-arterial abciximab. Initial angiographic results and clinical outcomes were analyzed.
Methods
We retrospectively reviewed the last 100 consecutive patients (examined between November 2002 and September 2003) with an intracranial aneurysm who were treated with coil embolization at our institution. Seven patients were identified by searching and reviewing the operative records in our database for the keywords thrombus or clot. The patients included five women and two men aged 45–71 years, four of whom had ruptured aneurysms, who had acute thrombus formation during the coil-placement procedure (Table).
Summary of anatomic and clinical results
The information documented in the medical records and imaging studies was reviewed by an interventionist who did not participate in the procedures during which the acute thrombus formed (J.K.S., Y.N., or A.B.). All angiographic images and procedural documents were then reviewed for the location, dimensions, and neck size of the aneurysm; for the type of coil and thrombolytic used, with the dose and route; for the activated clotting time (ACT) recorded closest to the intervention; and for angiographic and clinical results after intervention. Postprocedural cross-sectional images were also reviewed. The medical records of the patients were retrospectively reviewed for the rupture status of the aneurysm, and if it was ruptured, for the initial Hunt and Hess grade. Clinical outcomes were noted, and up-to-date clinical information was obtained from the outpatient assessment, when available.
All patients were treated under general anesthesia. Patients with unruptured aneurysms underwent systemic heparinization after arterial access was obtained. In most patients with ruptured aneurysms, systemic heparinization was delayed until after one or more coils were successfully placed. In general, patients received an intravenous bolus of heparin, approximately 50–100 U/kg followed by an infusion of 10–20 U/kg/h, adjusted to maintain an ACT (measured approximately every half hour) of greater than 300 seconds. In addition, pressurized flushing of the arterial sheath and guide catheter was done with 4000 U heparin per liter of sodium chloride solution.
The intravenous heparin infusion was continued after the procedure for 24–48 hours, with a target partial thromboplastin time of 50–70 seconds. In general, the heparin infusion was stopped immediately after the administration of abciximab, but the anticoagulation was not reversed in six of the seven patients. In one patient who also received intravenous abciximab, the systemic anticoagulation was partially reversed with intravenous protamine. In two cases, intravenous abciximab was also administered: one in which thrombus formed at the coil mass in conjunction with a more distal thromboembolism and another in which attempts to place a stent across a protruded coil loop were unsuccessful but caused vessel spasm. Postprocedural cross-sectional imaging was performed in all seven patients before discharge. In one case, only MR imaging was performed. In two cases, both MR images and CT scans were obtained, and in four cases, only CT scans were obtained.
Results
The Table summarizes the anatomic and clinical results. Local intra-arterial abciximab resulted in complete clot thrombolysis in four of seven patients. In two patients, complete proximal recanalization was achieved, but distal thromboembolic occlusion persisted. In one patient, distal thromboembolism did not respond to intra-arterial abciximab. In two patients, adjunctive treatment with intravenous abciximab bolus without infusion was performed (3 mg in case 4, 13 mg in case 3); no added angiographic effect was observed. ACTs measured closest to the time of abciximab administration were 231–340 seconds, with a mean of 285 seconds. In five of seven patients treated with intra-arterial abciximab, groin hemostasis was achieved with a suture-closure device (Perclose; Abbott Vascular Devices, Redwood City, CA) immediately at the end of the procedure. For two patients, the sheaths were sutured to the skin and removed several days later.
Six patients had a good clinical outcome at discharge. Up-to-date clinical follow-up was available in three patients who were seen in our outpatient clinic. For these three patients, no new neurologic deficits were documented. One patient with a grade IV condition eventually died from sequelae of the initial hemorrhage (withdrawal of care). For one patient, immediate postprocedural CT revealed extravascular contrast enhancement not documented angiographically during the procedure; however, no apparent change in the patient’s neurologic status was identified on physical examination. In one case, aneurysm rerupture occurred during the first coil deployment, as indicated by frank extravasation of contrast material. However, the hemorrhage was controlled with further coil placement, and thrombus formed adjacent to the coils. Despite the risk of further rehemorrhage, this thrombus was treated with intra-arterial abciximab, with recanalization, and the patient did well clinically.
In one patient with multiple aneurysms and an arteriovenous malformation, MR imaging was performed 2 days after embolization. T2-weighted images demonstrated an arteriovenous malformation with increased signal intensity and associated restricted diffusion in the splenium of the corpus callosum; this finding was considered to be secondary to cyanoacrylate embolization of the arteriovenous malformation within the brain. In the patient presenting with an aneurysm in the right middle cerebral artery bifurcation, CT demonstrated an infarct in the right frontal lobe without hemorrhagic transformation. Two patients with aneurysms of the anterior communicating artery had small areas of cortical ischemia. However, the ischemic changes documented on cross-sectional images did not lead to a new, clinically evident neurologic deficit.
Illustrative Case 1
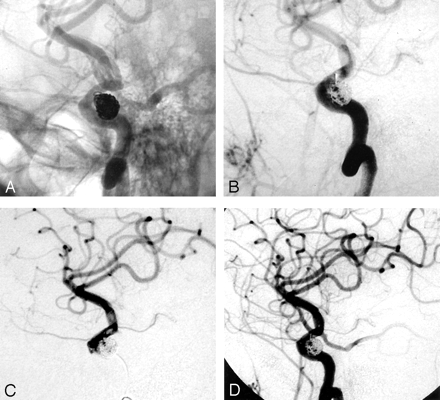
A 45-year-old woman with multiple sclerosis underwent MR imaging and MR angiography, which depicted a 7-mm, wide-necked (4-mm), right superior hypophyseal aneurysm. She then underwent elective coiling of her aneurysm. The patient was placed under general anesthesia, and a 7F sheath was placed in the right common femoral artery. The patient received systemic anticoagulation with intravenous heparin, with the ACT maintained over 300 seconds throughout the procedure. A 7F guide catheter (Vistabrite VBL; Cordis, Miami, FL) was selectively placed in the cervical segment of the right internal carotid artery in anticipation of balloon remodeling technique. A 7-mm × 21-cm coil (Trufill DCS complex; Cordis, Miami, FL) was first placed into the aneurysm without incident. This was followed by four other coils (Matrix; Boston Scientific; Natick, MA): one standard 2D 4-mm × 10-cm, one soft 2D 5-mm × 6-cm, one soft 2D 4-mm × 8-cm, and one soft 2D 4-mm × 8-cm. The last coil pushed one coil loop from a previous coil into the parent vessel (Fig 1A). After an additional Guglielmi detachable coil (GDC, soft 3 mm × 4 cm; Boston Scientific) was placed and the microcatheter removed, the coil loop protruded further into the parent vessel. A 20-minute delayed control angiogram revealed further protrusion of the coil loop associated with pulsation. A 3.5-mm × 12-mm stent (S7 AVE; Medtronic, Minneapolis, MN) was advanced to jail the coil loop. However, because of vessel tortuosity, the stent could not be advanced safely without causing substantial spasm, which was eventually relieved with intra-arterial nitroglycerin (100 μg). The coil loop was observed for over an hour without change. Follow-up angiography revealed slower flow through the right internal carotid artery and an increasing filling defect about the coil loop (Fig 1B). A microcatheter (Prowler14; Cordis) was then carefully navigated immediately proximal to the coil loop and adherent thrombus. From this location, 2 mg (in 2 mL) of intra-arterial abciximab was infused (Fig 1C). A control angiogram showed resolution of the thrombus (Fig 1D) without evidence of distal thromboembolism. The patient was awakened from general anesthesia and found to be neurologically intact.
Illustrative case 1.
A, Right internal carotid artery angiogram, oblique projection, unsubtracted, shows a coil loop protruding into the parent vessel.
B, Right internal carotid artery angiogram, oblique projection, subtracted view, shows a filling defect about the coil loop, indicating thrombus formation. Note the decreased antegrade flow into the supraclinoid internal carotid artery.
C, Right internal carotid artery angiogram obtained via a microcatheter injection. The image clearly shows the filling defects about the protruded coil loop and more distally.
D, After a local, low-dose (2-mg), intra-arterial infusion of abciximab through the microcatheter, thrombus dissolution is documented without evidence of distal thromboembolism.
Illustrative Case 2
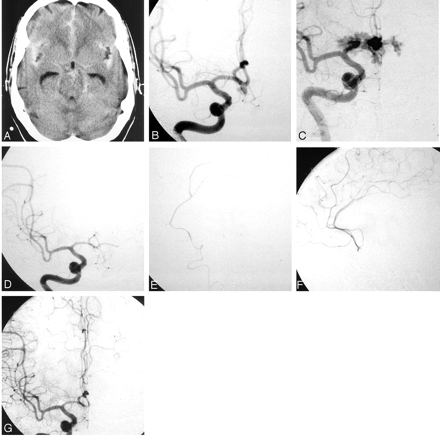
A 50-year-old woman presented with a grade I subarachnoid hemorrhage (Fig 2A). A 5-mm × 4-mm anterior communicating artery aneurysm was identified (Fig 2B). The patient was placed under general anesthesia, and a 5F sheath was inserted into the right common femoral artery. A 5F Envoy guide catheter was then selectively placed into the right internal carotid artery. A microcatheter (Prowler14; Cordis) was used to selectively catheterize the aneurysm, without incident. A GDC-10 (3D, 4 mm × 10 cm) was first deposited into the aneurysm. However, as the coil was advanced into the aneurysm during the last 2 cm of coil, a small coil loop was seen to extend beyond the confines of aneurysm roadmap. This correlated with an acute elevation in blood pressure, and control angiography showed extravasation of contrast material, a finding that indicates aneurysm re-rupture (Fig 2C). We continued coil placement, first pushing the rest of the first coil into the aneurysm. A second coil, GDC-10 (3 mm × 4 cm) was then advanced into the aneurysm sac. Control angiograms obtained approximately 4 minutes after the rupture showed a marked reduction in extravasation. Another control angiogram obtained 2 minutes later showed complete hemostasis. No further extravasation was identified. A third GDC-10 (soft 2 mm × 4 cm) was then placed into the aneurysm. At control angiography, filling defects were then identified adjacent to the coils; these were associated with marked delay in filling of distal anterior cerebral arteries bilaterally (Fig 2D). A microcatheter was navigated to the region of the coil mass and beyond, into the right proximal A2 segment of the anterior cerebral artery. A manual injection through the microcatheter revealed substantial thromboembolism (Fig 2E). Intra-arterial abciximab (5 mg) was infused via the microcatheter, resulting in recanalization of the entire anterior cerebral artery territory (Fig 2F). A final postembolization angiogram of the left internal carotid artery showed aneurysmal occlusion and normal filling of both anterior cerebral artery territories (Fig 2G). Postprocedural CT revealed extensive extravasation of contrast agent; however, no substantial mass effect was identified. The patient awoke without focal deficit. She was discharged home from the hospital. On follow-up clinic visitation, she was neurologically intact.
Illustrative case 2.
A, Nonenhanced CT scan shows subarachnoid hemorrhage and ventricular prominence.
B, Right internal carotid artery angiogram, frontal projection, shows a heart-shaped anterior communicating artery aneurysm pointing inferiorly, with filling of both anterior cerebral arteries through the anterior communicating artery.
C, Right internal carotid artery angiogram, frontal projection, shows frank extravasation of contrast material during deployment of the first coil.
D, Right internal carotid artery angiogram, frontal projection, obtained after coil placement inf the aneurysm shows no further extravasation of contrast agent. However, both anterior cerebral arteries no longer fill.
E, Right anterior cerebral artery angiogram, with a manual injection via a microcatheter, lateral projection, shows a paucity of branch filling consistent with extensive thromboembolism.
F, Right anterior cerebral artery angiogram, with a manual injection via a microcatheter, lateral projection, after local low-dose intra-arterial infusion of 5 mg of abciximab through the microcatheter, shows recanalization of the anterior cerebral artery branches.
G, Right internal carotid artery angiogram, frontal projection, after aneurysm rupture, aneurysm coil placement, and thrombolysis with intra-arterial ReoPro shows no filling of the anterior communicating artery aneurysm and normal filling of both anterior cerebral arteries.
Discussion
From November 2002 in our institutional analysis of over 100 consecutively coiled aneurysm patients, approximately 7% had identifiable thrombus formation during the procedure. In these seven patients, the thrombus was treated with intra-arterial abciximab, resulting in recanalization in most cases: four were complete, two were complete proximally with residual distal thromboembolism, and one resulted in no recanalization of the distal thromboembolism. In general, thrombus formation did not lead to increased periprocedural morbidity or mortality. Given the complexity of some of the cases, it is likely that management of the thrombus with intra-arterial abciximab contributed to the surprisingly favorable outcomes. In particular, intra-arterial abciximab was used in four patients with acutely ruptured, but protected, aneurysms without clinically important intracranial or systemic bleeding complications. In one case, low-dose intra-arterial abciximab was used to treat a thrombus immediately after an intraprocedural rerupture of an aneurysm, as documented by the frank extravasation of contrast material. (The aneurysm was first secured with coil placement.) In one case, postprocedural CT revealed contrast attenuation within the sulci that was not detected on cerebral angiograms during the procedure. Such leakage of contrast material without frank extravasation did not lead to neurologic deterioration; however, this finding accentuates the potential bleeding risk with glycoprotein IIB-IIIA inhibitors.
The local intra-arterial infusion of glycoprotein IIB-IIIA inhibitors is an addition to the options available to treat thrombi that form during neurointerventional procedures. Medical treatment with hypervolemia, hemodilution, increased intravenous heparin, periprocedural and postprocedural antiplatelet agents, intra-arterial thrombolysis with urokinase or tissue plasminogen activator, and intravenous bolus injection and infusion of glycoprotein IIB-IIIA inhibitors have been used to manage periprocedural acute thromboembolism (6–10). Cronqvist et al (8) used selective local intra-arterial infusions of urokinase to treat periprocedural thromboembolism in 19 patients undergoing coil treatment. They reported complete recanalization in 10 patients and partial recanalization in nine, with 14 patients eventually having good outcomes. However, in three of six patients with ruptured aneurysms, devastating intracerebral hemorrhage occurred. Derdeyn et al (14) treated with intra-arterial thrombolytics four of six patients (4.5% of 133 patients treated over 5 years) in whom intra-arterial thrombus formed during GDC placement. Three received urokinase and one received retevase, with good angiographic results; one of the four patients awoke with a new neurologic deficit. More recently, Workman et al (6) identified thrombus formation at the coil–parent artery interface in nine (4.3%) of 210 patients undergoing coil placement within a 30-month period. The thrombus was treated with additional intravenous heparin (five patients) and additional heparin with intravenous bolus injection and infusion of glycoprotein IIB-IIIA inhibitor (four patients), with good clinical results. However, the authors elected not to treat any of the four patients with ruptured aneurysms by using intravenous glycoprotein IIB-IIIA inhibitor.
The use of glycoprotein IIB-IIIA inhibitors, such as abciximab, in the setting of acutely ruptured aneurysms is untested. In one case report, an intravenous bolus of a glycoprotein IIB-IIIA inhibitor abciximab, without a 12-hour infusion was given to a patient with grade I subarachnoid hemorrhage; this led to a good clinical outcome (9). In our series, four patients with acutely ruptured aneurysms developed thrombus during coil placement and were treated with intra-arterial abciximab, with good angiographic results. All aneurysms were secured with coils before abciximab was infused.
Because acute thrombus is rich in platelets (white clot), glycoprotein IIB-IIIA inhibitors may be more effective than fibrinolytics, such as urokinase, in dissolving acute thrombus (8, 9). In one patient, intra-arterial abciximab failed to recanalize a distal thromboembolism, which was thought to be chronic, organized clot less responsive to glycoprotein IIB-IIIA inhibition. In this patient, a large aneurysm of the left middle cerebral artery bifurcation was initially coiled, which compacted 2 years later. The thromboembolic event occurred during the recoiling procedure and likely represented a fragment of organized clot that was dislodged from within the aneurysm during coil placement.
The reported risk of ischemic stroke after intracranial aneurysm coil placement is 1–17% (1–3, 5, 14). In analyzing postprocedural ischemic events after the treatment of intracranial aneurysms with GDCs, Derdeyn et al (14) estimated that the actuarial risk of stroke is 3.8%. On the basis of multivariate analysis, they identified a larger aneurysm diameter and protruding coils as significant independent predictors of postprocedural ischemic events after GDC placement, but they found no factor associated with intraprocedural intra-arterial thrombus formation. Their relatively low incidence of intraprocedural intra-arterial thrombus formation may reflect a more aggressive heparinization and antiplatelet protocol (14). The trend to use bioactive coils and other surface-modified coils might increase the risk of periprocedural and postprocedural thrombus formation and ischemic stroke (15–17).
The dose and safety profiles of glycoprotein IIB-IIIA inhibitors have been investigated in the prevention and treatment of thromboembolic and ischemic complications in coronary interventions (18, 19), but the optimal dose and delivery method in cerebrovascular interventions are unknown. The pharmacology of the intravenous bolus injection and infusion of abciximab in coronary intervention leads to the inhibition of platelets within 10 minutes and to the persistent blockage of glycoprotein IIB-IIIA receptors at a rate of 29%, even 8 days after administration (13). In several of our cases, immediate thrombolysis was achieved with relatively low dose, local, intra-arterial infusion of abciximab (2–5-mg intra-arterial bolus versus 15–25-mg intravenous bolus).
Glycoprotein IIB-IIIA inhibitors bind to the IIB-IIIA surface-membrane glycoprotein platelet receptor and primarily prevent platelet cross-linking and aggregation (13, 18, 19). In addition to preventing fibrinogen cross-linking and platelet aggregation, glycoprotein IIB-IIIA antagonists have other pharmacologic properties (20, 21). The glycoprotein IIB-IIIA antagonists can disaggregate newly formed platelet clusters in vitro, even when their potential fibrinolytic activity is ruled out. Because enzyme-dependent fibrinolysis does not appear to be involved in this setting, competitive removal of fibrinogen by the receptor antagonists is a likely mechanism to produce dispersal of the clot. Other mechanisms of action attributed to the platelet glycoprotein IIB-IIIA receptor antagonists that could potentially affect their efficacy in vivo are their ability to prevent fibrinogen-mediated aggregation and inhibit platelet-dependent prothrombinase activity and thrombin generation in a concentration-dependent manner. They may have some function as anticoagulants, and they may also promote fibrinolysis. The mechanisms are not well elucidated but may involve decreasing fibrin production, decreasing inhibition of rt-PA, or increasing urokinase production (18, 19, 22).
Although awareness of the use of intravenous glycoprotein IIB-IIIA inhibitors during neurointerventional procedures is growing, it should be emphasized that its intravenous use has been associated with higher risk of complications at the vascular access site, excessive bleeding if emergent surgery is needed, and thrombocytopenia, intracranial hemorrhage, and its effects are not easily reversed (11–13). Moreover, in the setting of aneurysm coil placement, emergency life-saving ventricular drainage may carry significant risk after an intravenous bolus and infusion of glycoprotein IIB-IIIA. To limit the periprocedural and postprocedural bleeding risk, a low-dose, local, intra-arterial infusion of glycoprotein IIB-IIIA inhibitors may have advantages over an intravenous administration in the emergency treatment of acute intracranial thrombus formation. Intra-arterial abciximab may allow higher local concentrations of glycoprotein IIB-IIIA inhibitor to bind the recently formed thrombus to maximize inhibition of platelet aggregation, to induce natural thrombolytics, and to inhibit release of thrombin from the recently formed clot. Theoretically, a lower intra-arterial dose may achieve the same local thrombolytic effect as that of an intravenous dose, and it may lead to a lower systemic level of receptor inhibition. This may decrease subsequent systemic bleeding complications. The thrombus dissolution and systemic platelet effects of intravenous and intra-arterial glycoprotein IIB-IIIA inhibitors should be directly compared in appropriate animal models. Direct comparisons with local, low-dose, intra-arterial infusions of other glycoprotein IIB-IIIA inhibitors with shorter half-lives should also be investigated as they may have further advantages in this setting (eg, peptide eptifibatide, Integrilin; MK-383, Aggrastat).
More extensive clinical experience is needed to validate the safe use of local, low-dose, intra-arterial glycoprotein IIB-IIIA inhibitors such a abciximab in cerebrovascular interventions. This may lead to an improved or optimal management regimen for treating thromboembolic complications, particularly in the setting of an acute aneurysm rupture.
Conclusion
Although no definite management recommendations can be made, and although more clinical assessment is needed to define its safety profile, our preliminary experience in a few patients suggests that a local, low-dose (2–5-mg), intra-arterial infusion of abciximab may be relatively safe and effective in achieving timely recanalization of an acute thrombus formed during intracranial aneurysm coil placement. The use of intra-arterial abciximab to treat acute thrombus formation was associated with relatively good clinical outcomes in patients with ruptured or unruptured aneurysms. Intra-arterial abciximab infusion may have less risk of postprocedural systemic bleeding than intravenous abciximab bolus and infusion.
References
- Received October 17, 2003.
- Accepted after revision January 1, 2004.
- Copyright © American Society of Neuroradiology