Abstract
BACKGROUND AND PURPOSE: Mental imagery involves rehearsing or practicing a task in the mind with no physical movement. The technique is commonly used, but the actual physical foundation of imagery has not been evaluated for the fast, complex, automatic motor movement of the golf swing. This study evaluated motor imagery of the golf swing, of golfers of various handicaps, by using functional MR imaging to assess whether areas of brain activation could be defined by this technique and to define any association between activated brain areas and golf skill.
METHODS: Six golfers of various handicap levels were evaluated with functional MR imaging during a control condition and during mental imagery of their golf swing. Two control conditions were evaluated—“rest” and “wall”—and were then subtracted from the experimental condition to give the functional activation map. These control conditions were then tested against the golf imagery; the participants were told to mentally rehearse their golf swings from a first person perspective. The percentages of activated pixels in 137 defined regions of interest were calculated.
RESULTS: The “rest-versus-golf” paradigm showed activation in motor cortex, parietal cortex, frontal lobe, cerebellum, vermis, and action planning areas (frontal and parietal cortices, supplementary motor area, cerebellum) and areas involved with error detection (cerebellum). Vermis, supplementary motor area, cerebellum, and motor regions generally showed the greatest activation. Little activation was seen in the cingulate gyrus, right temporal lobe, deep gray matter, and brain stem. A correlation existed between increased number of areas of activation and increased handicap.
CONCLUSION: This study showed the feasibility of defining areas of brain activation during imagery of a complex, coordinated motor task. Decreased brain activation occurred with increased golf skill level for the supplementary motor area and cerebellum with little activation of basal ganglia.
Mental imagery involves rehearsing or practicing a task in the mind with no physical movement. The technique is commonly used and widely advocated (Paula King, www.golfmagazine.com/fitness/clinic/stress/imagine.html; Leadbetter’s images, http://www.golfdigest.com/instruction/index.ssf?/instruction/leadbett_tdgzpkic.html). In golf, this has been most recently emphasized by the mental rehearsal techniques that professional golfer Phil Mickelson has publicly discussed (http://www.phil-mickelson.com/x755.xml). The actual physical foundation of imagery, however, has not been evaluated for fast, complex, automatic motor movements, such as the golf swing. This study evaluated motor imagery of the golf swing of golfers of various handicaps by using functional MR imaging to assess whether areas of brain activation could be defined by this technique and to define any association between activated brain areas and golf skill.
Methods
Six male golfers of various handicap levels were evaluated with functional MR imaging during a control condition and during mental imagery of their golf swing. The ages of the study participants ranged from 24 to 50 years, with an average age of 39 years. Five of the six participants were right-handed and played golf right-handed. The participant with a handicap of 5 was left-handed but played golf right-handed. The Cleveland Clinic Foundation Institutional Review Board approved the study. Written informed consent was obtained from all participants.
3D gradient-echo sequences were obtained of all participants for functional data fusion. Participants were instructed beforehand concerning the specific type of mental imagery to be used (ie, internal first person viewpoint) and the nature of the two control paradigms. Two control conditions were evaluated—“rest” and “wall”—and were then subtracted from the experimental condition to render the functional brain map. For the rest control paradigm, the participants were told to project themselves into a restful state, such as sitting quietly on a beach, taking care not to move mentally (or physically) during the study. For the wall control paradigm, the participants were told to imagine leaning against a wall with their hands outstretched and pushing against it. These control conditions were then tested against the golf imagery; the participants were told to mentally rehearse their golf swings from a first person perspective, as they would on a practice tee, with each swing occurring every 1.5 to 2 s. The imaged swing was to be full, as with a high iron or wood. Participants were told not to perform their usual pre-shot setup routines, in a effort to achieve a mental swing rate of approximately 0.5 swings/s. Data for the control and experimental conditions were alternately obtained during one single MR imaging series, such that while the images were acquired, the participant would alternate the imagery: rest, golf, rest, golf, etc., or wall, golf, wall, golf, etc. Participants were verbally told when to switch imagery tasks. MR imaging studies were obtained with the room lights dimmed and the eyes closed. The golf imagery was performed as the third series, after a dominant hand-alternating finger-tapping paradigm and an imagined finger tapping paradigm, to allow the participant to become accustomed to the testing procedures.
Blood oxygen level-dependent functional studies were performed on either a 1.5-T whole body MR imaging system (Symphony, Siemens, Erlangen) or a 3-T head only MR imaging system (Allegra, Siemens, Erlangen) (1). With the 1.5-T system, the body coil was used to transmit, with a receive-only head coil collecting the data. A transmit/receive head coil was used to acquire the 3-T functional studies. All functional images were acquired with by using a 2D multisection gradient-echo echo-planar imaging acquisition. Sixteen section locations were collected every 3 to 4 s with fat saturation. The acquisition parameters were as follows: 1442/50 (TR/TE); excitation flip angle, 90 degrees; field of view, 220 mm; matrix = 64 × 64; phase, R/L; section thickness, 4 mm with a 33% gap. The functional images were acquired in the axial or oblique transaxial plane, positioned to cover the entire cortex and most of the cerebellum. Signal intensity bandwidth was 1953 Hz/pixel.
Prospective and retrospective motion correction was used for all functional MR imaging studies. The former was implemented primarily to minimize the effect of through-plane patient motion that typically occurs during a functional MR imaging study. The prospective motion correction was accomplished in real time by applying a 3D rigid body transformation to the most recent image volume against a reference volume (2). The new positional information is passed to the measurement system that uses these data to adjust the section orientation and position acquisition parameters for the next acquisition, allowing a constant spatial relationship between the measurement-head coordinate system. The updated positional information has a temporal delay of one acquisition cycle. Volume-to-volume motion that remains as a result of this temporal delay is corrected by the addition of retrospective motion correction of the volumes by using a k-space interpolation.
Statistical evaluation of the functional MR imaging data was conducted by automatic calculation of z maps. The calculated maps were then imported into Analyze 3.1 (Mayo Foundation), and regions of interest were drawn by hand around 137 different areas for both right and left cerebral and cerebellar hemispheres over the 16 brain sections: frontal, temporal, parietal, occipital, supplementary motor area, cingulate, motor, sensory, deep gray matter, cerebellar hemispheres, brain stem, and vermis. The percentage of activated pixels in each region of interest was then calculated. For one participant (13 handicap), the rest versus golf imagery was repeated five times and the data were summed to provide a template to allow comparison with the other participants. This participant was also evaluated on both 3.0- and 1.5-T systems for comparison of data quality.
Functional MR images were coregistered to the 3D gradient-echo images by using in-house software based on the method of maximization of mutual information (3). Mutual information is a statistical measurement of similarity between the data elements of two images in which neither fiducials (frame or scalp) nor preprocessing (brain segmentation) are required for calculating a coregistration transform. The functional MR image was linearly transformed by using six parameters and tri-linearly interpolated to match the dimensions and resolutions of the 3D gradient-echo image.
Interactive 3D surface reconstructions of the 3D MR images were generated by using real-time, hardware-based volume rendering on a Silicon Graphics, Inc. Onyx Infinite Reality computer (Mountain View, CA) with in-house developed software (4). A brain segmentation preprocessing step was necessary to mask all nonbrain structures as transparent for subsequent reconstructions. Remaining brain structures were rendered by using intensity- and opacity-based windowing algorithms as a piecewise linear function of MR imaging signal intensity (5). The coregistered functional MR image was then fused to the 3D MR surface reconstruction as pseudocolored voxels.
At the end of the MR imaging session, participants completed a validated Movement Imagery Questionnaire of Hall and Pongrac for evaluation of their perceived ability to perform mental imagery (6). The questionnaire grades visual and kinesthetic imagery on a 1- to 7-point scale, with 1 representing worst and 7 representing best.
Results
Five of the six participants completed both the rest-versus-golf and wall-versus-golf paradigms. The participant with a handicap of 10 completed only the rest-versus-golf portion. Four of the participants were evaluated on the 1.5-T system, and the participants with handicaps of 7 and 11 were evaluated on the 3.0-T system. The mean score for the Movement Imagery Questionnaire was 5.59 (range, 4.63–6.38).
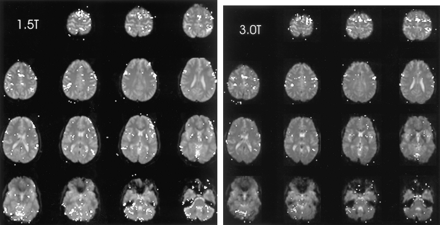
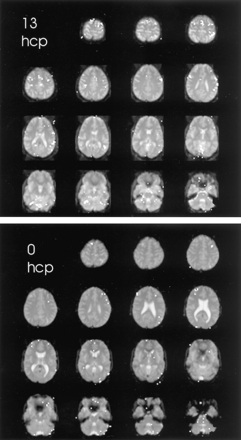
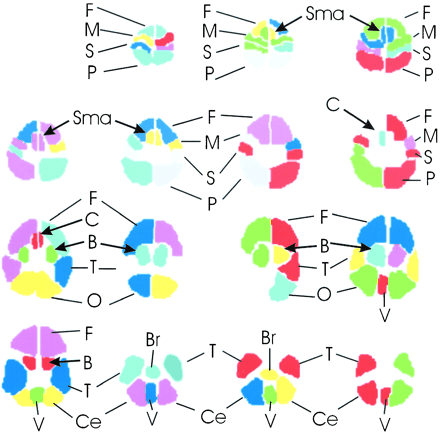
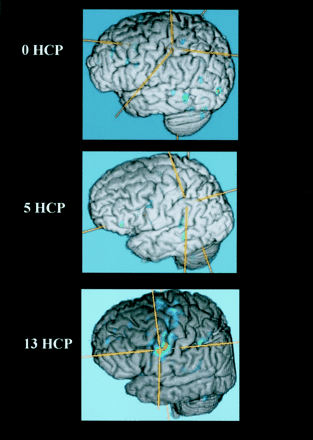
The brain functional map of the rest-versus-golf paradigm for both 1.5 and 3.0 T for the participant with a handicap of 13 is shown in Figure 1. The regions of activation are similar between the two systems, with 2.7% of pixels showing activation at 1.5 T and 3.8% at 3.0 T. The functional maps of the wall-versus-golf paradigm for the participants with handicaps of 0 and 13 are shown in Figure 2. Schematics of the regions of interest drawn for each participant with labels are shown in Figure 3.
Areas of brain activation for the participant with a handicap of 13, rest-versus-golf paradigm for 1.5 T (left) and 3.0 T (right), show a similar pattern for both field strengths. Areas of brain activation are shown overlaid on the axial view images of the brain.
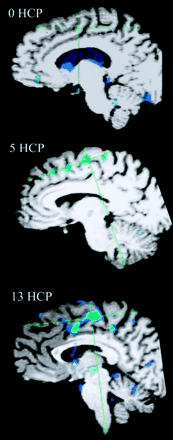
Areas of brain activation compared for the participants with handicaps (hcp) of 13 (upper panel) and 0 (lower panel), wall-versus-golf paradigm. Image of the participant with a handicap of 13 can also be compared with the other paradigm shown in Figure 1. The wall-versus-golf paradigm shows overall diminished brain activation, with much less activation in the better player.
Schematic of regions of interest drawn for each participant. The brain regions are shown for each typical section of the functional MR imaging data set, proceeding as in Figures 1 and 2 from the top of the brain at top left to the base of the brain at bottom right. F, frontal lobe; M, motor; S, sensory; P, parietal; Sma, supplementary motor area; C, cingulated; B, basal ganglia; T, temporal lobe; O, occipital lobe; Ce, cerebellar hemisphere; Br, brain stem; V, vermis of cerebellum.
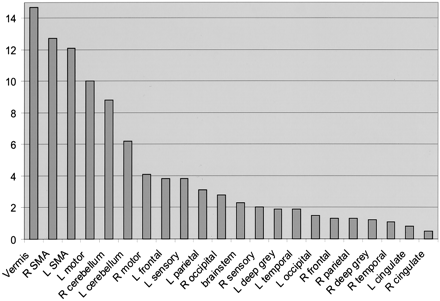
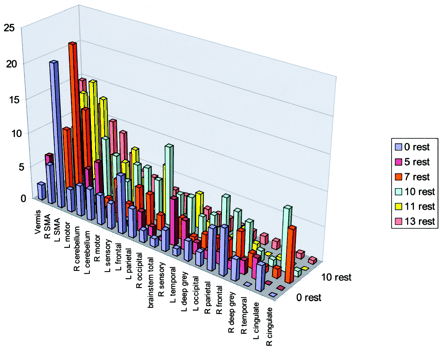
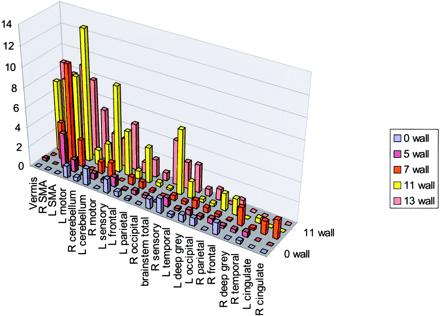
The five functional MR imaging sessions of the participant with a handicap of 13 were used as the template for reference for the other four participants. For the participant with a handicap of 13, areas of activation from greatest to least are shown in Table 1 in boldface type and in Figure 4. Percentages of activation for all areas are presented for both experimental conditions—rest versus golf and wall versus golf—in Table 1 and Figure 5. Percentage of activation for the wall-versus-golf paradigm is presented in Figure 6.
Percent area of activation in the participant with a handicap of 13. Data shown represent an average of the percentage area of activation from five separate experiments of the rest-versus-golf paradigm and are sorted from the highest level of activation to the lowest. Vermis of cerebellum, supplementary motor area, motor areas, and cerebellar hemispheres show the greatest activation. R, right; L, left; SMA, supplementary motor area.
All data from the rest-versus-golf paradigm are sorted by areas of activation using the data from the participant with a handicap of 13 from the rest-versus-golf paradigm as a template. R, right; L, left; SMA, supplementary motor area.
Wall-versus-golf paradigm data sorted by areas of activation using the data from the participant with a handicap of 13 as a template, as in Figure 5. R, right; L, left; SMA, supplementary motor area.
Summary of percentage of activation for both experimental conditions*
The rest-versus-golf paradigm showed activation as follows: in the region of the primary motor control (motor cortex); in areas concerned with imagery generation (parietal cortex), especially left parietal lobe; in areas concerned with execution (frontal lobe, cerebellum, vermis); in action-planning areas (frontal and parietal cortex, supplementary motor area, cerebellum); and in error detection (cerebellum). Vermis, supplementary motor area, cerebellum, and motor regions generally showed the greatest activation. Very little activation was seen in the cingulate gyrus, right temporal lobe, deep gray matter, and brain stem.
The wall-versus-golf paradigm showed generally diminished activation across all regions, compared with the rest-versus-golf paradigm. Almost no regions showed increased activity in the wall-versus-golf paradigm compared with the rest-versus-golf paradigm across participants, with the exception of small increases in the right and left sensory strips and brain stem for the participant with a handicap of 13. The wall-versus-golf paradigm showed decreased activation diffusely but most extensively with the better players.
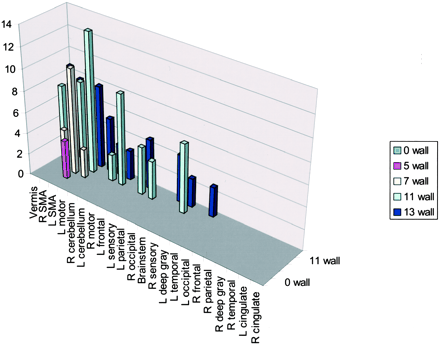
A correlation existed between increased number of areas of activation and increased handicap if 2% activation is considered the cut-off for system noise (Table 2, Fig 7). This activation again was mainly present in the supplementary motor area, vermis, cerebellum, and motor areas. Fusion of the 3D T1-weighted images and functional data along lateral and mesial brain surfaces for a variety of handicaps are shown in Figures 8 and 9.
Activation of wall-versus-golf paradigm of ≥2%. The greatest activation in multiple areas occurred in the participant with the highest handicap. R, right; L, left; SMA, supplementary motor area.
Fused functional and 3D T1 gradient-echo data for three participants spanning the handicap (HCP) range show increased activation along the motor cortex in the participant with the highest handicap.
Fused functional and 3D T1 gradient-echo data show mesial surface of right hemisphere, with increasing supplementary motor area activation correlated with increasing handicap (HCP).
Summary of percentage of activation ≥2%, wall-versus-golf paradigm
Discussion
Mental imagery is experience that resembles perceptual experience but occurs in the absence of the appropriate stimuli for the relevant perception (7). Imagery is considered to play a pivotal role in memory and motivation experiences (8). The capacity of the nervous system to simulate behavior of the motor system is an important issue in studies of motor control and mental processes (9). Imagery can be used to improve competitive performance in sports or to induce sensorimotor plasticity through mental rehearsal (10–12).
This study showed the following: 1) defining brain areas of activation by using functional MR imaging in complex motor imagery is feasible; 2) decreased brain activation occurred with increased golf skill level for the supplementary motor area and, in particular, the cerebellum (vermis); 3) golf swing motor imagery produced little activation of cingulate gyri or basal ganglia across all skill levels; 4) the wall-versus-golf paradigm seems to offer more discriminatory power in areas of activation compared with the more general activation of the rest-versus-golf paradigm.
We found generally good agreement between golf swing imagery and brain activation areas defined in the literature, including primary motor control (motor cortex), imagery (parietal cortex), execution areas (premotor cortex of frontal lobe, lateral cerebellum, basal ganglia, vermis, and medial cerebellar hemispheres), action-planning areas (frontal and parietal cortices, supplementary motor area, lateral cerebellum), and error detection (cingulate, cerebellum). Although most of these areas did show activation with golf swing motor imagery in this study, notably absent was cingulate or basal ganglia activation. The role of the basal ganglia in advanced stages of learning is uncertain (13). Activation of the basal ganglia has been shown for early learning but not for late learning by some investigators (14, 15). Other investigators have not seen significant activation in the basal ganglia related to practice (16).
We identified activation in the participants with higher handicaps to particularly involve vermis, supplementary motor area, and motor cortex. The motor cortex involvement seems straightforward for imagery that involves so many muscle groups. Studies have shown supplementary motor area involvement with performance of self-initiated, internally cued tasks, particularly tasks of a sequential/temporal nature or those that are overlearned (17, 18). Grafton et al (19) reported an increase in supplementary motor area activation when participants improved performance to keep a stylus on a rotating disk, and van Mier et al (16) showed higher supplementary motor area activation during more skilled conditions than during unskilled performance. In contrast, Jueptner et al (15) reported increased supplementary motor area activation with new learning compared with control, versus prelearned sequence compared with control. Supplementary motor area activation is higher with more complex sequential tasks and with highly overlearned performance of complex sequential sequences (18, 20).
Decety et al (21) showed cerebellar activation that is not necessarily associated with actual movement, with activation of the middle and caudal parts of cerebellum bilaterally when participants imaged a tennis game. Deiber et al (22) showed that patients did not activate cerebellum when imaging simple finger-tapping tasks. They suggested that “executive” processes, such as execution of the motor task, activate vermis and medial regions of the anterior lobe whereas the lateral cerebellum plays a role in programming complex actions. Although many studies have shown a change in cerebellar activation as an effect of practice, the direction of change can vary. It has been conceived that early and intermediate stages of learning are accompanied by decreased cerebellar activation, whereas highly overlearned or automatic performance (such as the golf swing) coincides with increased activation (13).
A key assumption of this research is that mental rehearsal is somehow analogous to the motor planning that occurs with natural movements performed without thought. This assumption is supported by a variety of experiments (17, 23). Recent imagery findings have shown that the patterns of cerebral activation during the mental rehearsal of a motor act are similar to those produced by its actual execution (24). This fits the concept that some portion of the neural activity that takes place during movement involves internal simulations (25–30).
Previous functional MR imaging studies have shown that pixels activated during contraction of intrinsic hand muscle are activated during imagery of a movement involving the same muscle (30, 31). Confirmation of these results can also be found by using transcranial magnetic stimulation (28, 32, 33). In normal participants, brain activation during motor imagery depends on the hand used in the imagined movement (34).
Studies of various pathologic abnormalities have contributed to the understanding of areas involved in motor imagery. Sirigu et al (35) showed that a patient with hemiparesis related to cortical degeneration limited to primary motor cortex was still able to generate motor imagery with the affected hand. Simulated movements are slow in patients with Parkinson’s disease, as with executed movements (36). Patients with lesions only in the parietal cortex were found to be selectively impaired at predicting with mental imagery the time necessary to perform differentiated finger movements, suggesting that the parietal cortex is important in mental movement representations (37).
The particular paradigm used is obviously critical in determining the results. In imagery, there can be visual imagery, motor imagery, or a combination of both. This distinction is critical, because visual imagery suggests passively viewing a scene in your mind whereas motor imagery is “getting inside” your body to mentally perform the motor task in the first person. In this study, two primary control conditions were tested against golf imagery: rest and wall. The rest condition was chosen to allow visual imagery, with no kinesthetic or motor imagery, thus sitting quietly at the beach. The wall condition was chosen to bring into the equation imagery of all four extremities with a static motor component, purposefully eliminating any motor sequencing or timing effects. The subtraction, therefore, of these two paradigms from the golf motor imagery would be expected to show considerable difference. The rest-versus-golf study would be a relatively pristine map of all areas activated with the motor imagery, including static and dynamic aspects. The wall-versus-golf study would include only dynamic motor imagery aspects, including gross motor imagery, but also timing, rhythm, and direction. A fairly striking decrease in activation was observed across all areas with the wall-versus-golf paradigm, most conspicuous with the better players.
This study had several limitations. It was not possible, concurrent with the actual experiment, to determine the degree of mental performance while the experiments were running. This limitation is inherent with the technique of imagery which by definition is an internal mental construct. Monitoring for unwanted external motion does not answer the central question of what degree of internal “work” the participant is really performing, although such monitoring is necessary to exclude artifacts from real physical motor activity. In this study, an attempt was made to minimize variability by giving strict instructions to each participant regarding the specific task to be performed and by monitoring each participant in the magnet during each sequence for overt physical motion. In addition, a validated imagery survey was completed for each participant immediately after the examination, which showed a mean of 5.59 on a scale of 1 (worst) to 7 (best). No participant had a score <4.5, which means that no participant thought he was poor in visual or kinesthetic imagery. The number of participants evaluated for this pilot project was small, and further validation with a larger sample size is needed. Some variation in field strength of the systems used was observed, with two of six participants evaluated on the 3.0-T system. One participant was tested on both systems and showed a similar pattern of activation (Fig 1). Also, even excluding the 3.0-T data, the trend of increased activation with increasing handicap persists.
How brain activity relates to practice and learning is complex and, apparently, multifactorial. Studies have shown activation in brain areas to decrease, increase, or shift as an effect of repetition or practice. These apparently disparate results and variations could be due to the amount of practice the participant has had, the specific task involved, and whether the task is overlearned (38).
This study showed feasibility of defining areas of brain activation during imagery of a complex, coordinated motor task. This study also highlighted important follow-up areas of investigation: Does the correlation of increased areas of activation for the wall-versus-golf paradigm hold for handicaps >13? Can an imagery difference be shown in players who, to use the terminology of the movie The Legend of Bagger Vance, have “lost their swing”? Most importantly, can intervention (such as the use of relaxation techniques or self-hypnosis) change brain activation with concomitant improvement in performance (12)?
The presence of increased brain activation with poorer performance could have important implications for golf learning theory. The presence of increased activation for players high handicaps could potentially relate to two effects: 1) increased activation reflects a failure to learn and become highly automatic, or 2) increased activation is essentially pathologic and related to a loss of automaticity with compensatory increased brain activity. Development of automaticity is relative and can be dynamic and reversible. A classic example of this pathology is writer’s cramp (focal dystonia), with which the severe functional disturbances can be explained in terms of a loss of automaticity and an increased need for controlled processing (39). If the first scenario is correct, then poor performance could be improved by conventional teaching methods. If the second scenario is correct, and the brain activation reflects the need for compensatory processing, this would require more radical methods to measurably improve performance, because dystonic states typically are resistant to change.
Conclusion
This study showed the feasibility of defining areas of brain activation by using functional MR imaging during imagery of a complex, coordinated motor task. Decreased brain activation occurred with increased golf skill level for the supplementary motor area and, in particular, for the cerebellum (vermis). Golf swing motor imagery produced little activation of cingulate gyri or basal ganglia across all skill levels. The wall-versus-golf paradigm seems to offer more discriminatory power in areas of activation compared with the more general activation of the rest-versus-golf paradigm.
References
- Received September 13, 2002.
- Accepted after revision November 26, 2002.
- Accepted after revision November 26, 2002.
- Copyright © American Society of Neuroradiology