Abstract
Summary: Sacral insufficiency fracture is a debilitating injury for which no active treatment is currently available. We present three consecutive cases of elderly patients with sacral insufficiency fractures whose symptoms were relieved immediately by treatment of the fractures by polymethylmethacrylate injections, a so-called sacroplasty.
Sacral insufficiency fractures are a relatively common and debilitating source of lower back pain. Standard treatment requires prolonged bed rest, with the associated complications that result from immobility (1). Vertebroplasty is an increasingly popular technique of injecting polymethylmethacrylate cement into a vertebral compression fracture for pain relief and support. If these techniques could be applied to sacral insufficiency fractures, patients might gain some welcome relief. We present three consecutive cases of elderly female patients with documented sacral insufficiency fractures, whose sacral pain greatly diminished after treatment with polymethylmethacrylate injection, a so-called sacroplasty.
Case Reports
Case 1
A 76-year-old woman was referred for vertebroplasty for intermittent chronic lower back pain recently aggravated by a fall. The severity of her pain necessitated a wheelchair for mobility. During examination, she reported tenderness over L4, but her severe sacral pain dominated her presentation. Although a pelvic radiograph (Fig 1A) was normal, bone scintigraphy (Fig 1B) revealed the classic H sign of a sacral insufficiency fracture in addition to an active L4 compression fracture. As a planning step, preoperative pelvic CT showed sclerotic changes with cortical disruption bilaterally, confirming the sacral insufficiency fractures (Fig 1C). In addition to L4 vertebroplasty, she consented to attempt the sacroplasty alternative.
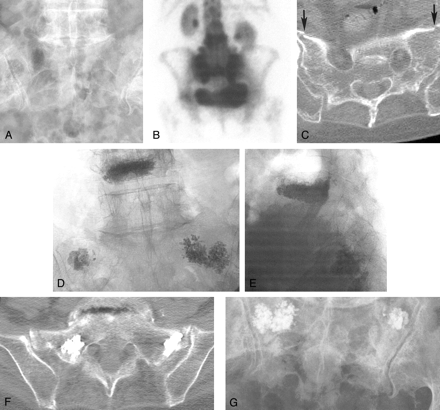
Case 1, a 76-year-old woman with low back pain.
A, Pelvic radiograph shows degenerative lumbar changes. Note, however, that the sacrum is unremarkable.
B, Bone scan shows the H sign diagnostic of a sacral insufficiency fracture. Prominent renal activity on the scan is a normal finding.
C, Cortical disruption (arrows) on a non-contrast-enhanced pelvic CT scan confirms fracture.
D and E, Anteroposterior (D) and lateral (E) fluoroscopic images show cement in the L4 vertebral body and cement bilaterally in the sacral ala. The lateral view illustrates the difficulty with visualization within the sacrum during this technique.
F, Postoperative pelvic CT scan shows cement within the bilateral superior sacral ala in the vicinity of the fracture lines
G, Follow-up pelvic radiograph shows the cement within the superior sacrum
After L4 vertebroplasty with standard prone positioning under conscious sedation with fentanyl and midazolam, the fluoroscopy unit was placed obliquely to provide access parallel to the sacroiliac joint. Biplane fluoroscopy was used to guide the needle into the sacrum, although the lateral view was limited in its usefulness for position confirmation. Using the Parallax injection system (Ezflow Cement Delivery System, Parallax Medical Inc., Scotts Valley, CA), we injected the polymethylmethacrylate cement and barium powder mixture under fluoroscopic monitoring (Fig 1D and E). The overlapping bony pelvis obscured sacral visualization, complicating our assessment of whether the injected cement was contained within the sacrum. A postoperative pelvic CT scan showed cement within the bilateral superior sacral ala, in the vicinity of the fracture lines (Fig 1F). Postoperative instructions included remaining in a supine position for 4 hours, with discharge home afterward.
Pain relief was immediately evident that night, with virtually complete relief of symptoms. At a 1-month follow-up clinical visit, she remained pain free, had regained her independence from a wheelchair and walker, and no longer needed analgesic medication. A follow-up pelvic radiograph showed the cement within the superior sacrum (Fig 1G). By telephone report 16 weeks after the procedure, the patient continued to be pain free with no recurrence of lower back complaints despite two minor falls at home in the interim.
Case 2
A 71-year-old woman had recurrent sudden-onset lower back pain that she rated 10 out of 10 for several months since falling. This acute pain differed from her chronic lower back pain. She relied on a walker for mobility. MR imaging and bone scintigraphy performed at another institution showed an old T11 compression fracture and bilateral sacral insufficiency fractures. Vertebroplasty at T11 was performed first, although her pain distribution was more clearly related to the sacral area. The subsequent sacroplasty was similar to that performed in our first patient, although venography was attempted for assessing needle placement (Fig 2A and B). Again, visualization of cement within the sacrum was difficult by fluoroscopy alone, and, in retrospect, the volume of cement in each of these first two cases (2–6 mL) was conservative because of increasing operator uncertainty regarding needle location (Fig 2C and D). A postoperative pelvic CT scan showed cement within the left sacrum, but the right sacral injection resulted in a portion of the cement being positioned within the posterior soft tissue (Fig 2E). Pain relief was immediately apparent by the time of discharge, later that same day, and was sustained through her 1-month follow-up clinic visit. She discontinued her pain medications. Her general activities of daily living were much improved and were described to be back to baseline by herself and her family members. In telephone follow-up with the patient 14 weeks after the procedure, she reported she was “wonderfully improved” and pain free in her lower back.
Case 2, a 71-year-old woman with sacral insufficiency fracture.
A and B, Anteroposterior (A) and lateral (B) venograms do not clearly confirm needle tip placement within the sacrum. Again, notice the difficulty in confirming the needle tip location as being entirely intraosseous.
C and D, Anteroposterior (C) and lateral (D) fluoroscopic images show cement within the sacrum.
E, Postoperative pelvic CT scan shows cement within the left sacrum, but the right sacral injection has resulted in a portion of the cement being positioned within the posterior soft tissue
Case 3
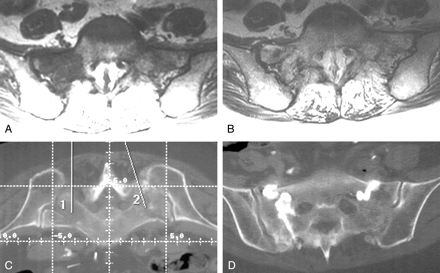
MR images obtained in a 74-year-old bedridden woman with severe lower back pain (Fig 3A and B) revealed edema within the sacrum, consistent with a sacral insufficiency fracture. Sacroplasty was performed in the CT suite, which greatly simplified needle placement (Fig 3C). Needle placement in the sacrum was performed by using CT guidance. A portable C-arm fluoroscope was brought to the CT suite and used to monitor cement injection. Postoperative CT confirmed the sacral cement placement (Fig 3D). The patient’s pain greatly diminished after the procedure, but she was lost to follow-up.
Case 3, a 74-year-old woman with sacral insufficiency fracture.
A and B, Preoperative sacral MR images show low-T1 (A[TR/TE, 450/14]) and high-T2 (B[4000/99]) signal intensity, consistent with the edema from a sacral insufficiency fracture.
C, Pelvic CT scan illustrates the two intended needle paths, simplifying needle placement during sacroplasty.
D, Postoperative CT confirms cement location.
Discussion
Although relatively common, sacral insufficiency fractures were only recently described by Lourie in 1982 (2). Osteoporosis is the leading cause, most often afflicting elderly women; other causes include chronic steroid use, radiation exposure, and arthritis. Patients complain of lower back pain that is often acute and may be associated with minimal trauma. These nonspecific symptoms and radiographic findings that are often normal make diagnosis elusive and potentially unrecognized. CT scans can show increased sclerosis and cortical disruption, whereas MR images can show marrow edema. A more sensitive tool, bone scintigraphy, shows increased activity, occasionally displaying an H pattern diagnostic of a sacral insufficiency fracture (3).
Traditional therapy for sacral insufficiency fractures requires extended bed rest with anesthesia. This therapy leads to complications of immobility: deep venous thrombosis, pulmonary embolus, muscle atrophy of 1–3% per day, impaired cardiac function, decubitus ulcers, poor pulmonary clearance, bone demineralization, and psychological changes. Early ambulation may reduce the complication rate (4).
Vertebroplasty involves injecting polymethylmethacrylate cement into vertebral bodies for stabilization. This technique has been successfully applied to vertebral angiomas, vertebral compression fractures, and malignant vertebral tumors (5–8). A natural extension of this technique is sacral insufficiency fractures.
Our three sacroplasties provided marked pain relief, improved activities of daily living, and reduced reliance on pain medication. Stabilization of the pelvis around the fracture site likely contributes to pain relief. Although concomitant vertebroplasties were performed for two of the three patients, their pain distribution was more clearly focused on the pelvis, implying that the pain relief was less likely from the vertebroplasty. The degree of pain relief did not appear to be necessarily proportional to the small amount of cement injected (2–6 mL). Similar results have been found with vertebroplasty (9–11). Although placebo effect may be a contributing factor, as is the case for vertebroplasty, it is very unlikely that placebo effect would account for the compelling degree of relief in three unrelated patients.
Preoperative planning requires diagnosis of a unilateral or bilateral sacral insufficiency fracture as the cause of low back pain. In light of the low sensitivity of radiography, bone scintigraphy is a recommended early diagnostic step (12). Normal bone scintigraphy findings exclude a sacral insufficiency fracture as the cause of pain.
The technical challenges of this procedure in the sacrum relate to safe placement of the needle and safe extrusion of the cement. Two of our three cases were done entirely with fluoroscopy. Using fluoroscopy alone for needle guidance in the sacrum proved extremely challenging, because it was very difficult to know whether the tip of the needle had adequately traversed the outer cortex and had not breached the inner cortex on the pelvic side. Furthermore, the injection of cement gave no sense of resistance in any of these cases, because the cement extruded easily into the capacious crevices of the sacral fracture. Although this is a very desirable outcome from a therapeutic point of view, it means that the operator had no feedback from the injector system as to whether the cement might be extruding into the soft tissues of the buttock or pelvis. To help confirm our needle positioning in the second case, venography was attempted, but it proved unhelpful (13). Because of our limited experience with this procedure, we recommend CT guidance for needle placement; CT might enable greater accuracy for locating the needle tip within the bone adjacent to the fracture zone. In the third case, CT was used for needle placement and fluoroscopy for injection, which proved very satisfactory. A CT fluoroscopy suite would be the ideal environment, providing accurate needle visualization and real-time monitoring of cement injection. In our third case, the portable C-arm fluoroscope simulated this ideal situation. CT alone is insufficient for sacroplasty in our opinion, because the real-time monitoring offered by fluoroscopy is necessary for early detection of cement migration.
The other concern during this procedure is extrusion of cement into the sacral foramina that poses risk of injury to the sacral nerves or cement migration into the spinal canal. Therefore, it seems prudent to focus on the upper outer ala of the sacrum at the time of needle placement to reduce the risks of cement migration in a hazardous direction. This might be a particular risk when a gaping fracture line is seen extending into a sacral foramen or into the dural canal on the preoperative CT scan. In view of our limited experience with this technique, we are not in a position to state whether such a finding at pretreatment CT represents an absolute contraindication to this technique. In our opinion, such findings should at the least suggest the use of extreme caution during cement injection.
In our three cases, no significant complications occurred. In one case, a small amount of cement entered the posterior soft tissue, without any notable complications for the patient. Like vertebroplasty, cement migration could cause venous emboli or neural compromise. These potential complications necessitate real-time monitoring of injections and cautious cement application.
Conclusion
The long-term results of sacroplasty are not known, but these few patients reported immediate relief of symptoms, and the procedure appeared to improve their quality of life dramatically. Patient satisfaction was achieved, and the potential complications of immobility were avoided. These three promising early results suggest that sacroplasty may be an alternative therapy for sacral insufficiency fractures that warrants further consideration.
References
- Received July 1, 2002.
- Accepted after revision August 7, 2002.
- Copyright © American Society of Neuroradiology