Abstract
Summary: A patent foramen ovale (PFO) is a fairly common septal defect in the general population. Paradoxical embolization through a PFO is a known cause of stroke. Preprocedural recognition of a PFO in a patient undergoing particulate arterial embolization can help reduce the risk of cerebral infarction.
A patent foramen ovale (PFO) is a common cardiac anomaly. The incidence of PFOs in autopsy studies are reported to be 12–35%, and clinical reports of patients younger than 55 years state that unexplained stroke due to PFO occurs in 12–41% of the population studied (1, 2). Large defects, those 0.6–1.0 cm, occurred in 6% of the autopsied group (1). When particulate emboli are injected into the arterial system to embolize a lesion that contains an arteriovenous shunt such as a tumor, arteriovenous malformation, or arteriovenous fistula, the interventionalist faces the risk of causing paradoxical infarction in the presence of a PFO. Knowing that such a cardiac abnormality is present can permit the physician to alter the treatment plan.
Case Report
A 14-year-old right-handed boy presented with chronic nasal congestion. MR imaging revealed a contrast-enhancing mass in the nasopharynx consistent with a juvenile nasopharyngeal angiofibroma (JNA). Prior to surgical resection, preoperative embolization was requested to reduce intraoperative blood loss.
In view of the patient’s age and inability to cooperate with the embolization procedure, he was placed under general endotracheal anesthesia. Neurophysiologic monitoring of bilateral somatosensory evoked potentials (SSEP) and electroencephalography (EEG) were performed. Arterial access was obtained via the right femoral artery by using a 6F sheath. A 6F guiding catheter was advanced into the right common carotid artery, and arteriographic series were performed. These studies demonstrated supply to the mass via the right internal maxillary (RIMax) artery, right ascending pharyngeal artery, and a branch from the anterior genu (C3 segment) of the right cavernous internal carotid artery. The guiding catheter was advanced into the right external carotid artery, and the RImax and right ascending pharyngeal arteries were sequentially catheterized by using a 0.018-inch microcatheter. Angiograms demonstrated no clear connections to the intracranial circulation. Methohexital (4 mg) was injected into each vessel prior to embolization, and no SSEP or EEG changes were seen. The mass was next embolized by using 300–500-μm Embospheres (Biosphere Medical, Rockland, MA) until early reflux was seen. The vessels were then permanently occluded with platinum coils. At the end of the procedure, the mass obtained its vascular supply from the cavernous carotid alone.
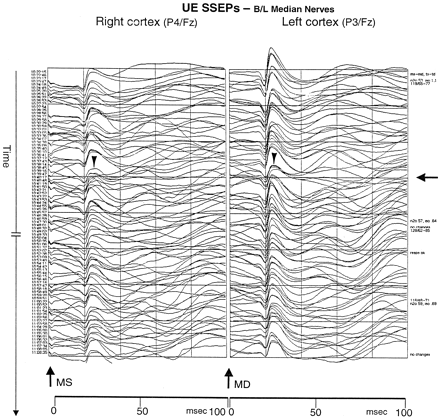
During the initial embolization of the RIMax artery, the neurophysiologist reported left hemispheric changes in the SSEPs and then right hemisphere changes. In both hemispheres, SSEPs were reduced by approximately 50%, with the left hemisphere slightly worse than the right. These changes then stabilized and improved slightly throughout the remainder of the procedure (Fig 1). Because no changes in arterial blood pressure or anesthetic agent could explain the neurophysiologic alterations and because the left hemisphere was affected during RIMax arterial embolization, we believed that the SSEP changes might have been technical. The procedure was completed. No supply arose from the left carotid system.
SSEP tracing demonstrates changes in recordings during the embolization. Upper extremity (UE) SSEP were obtained from stimulating the left (MS) and right (MD) median nerves at the wrists and by recording evoked potentials from the scalp. The arrow on the far right indicates the time at which the SSEP waveforms began to deviate. The arrowheads indicate the wave change with a 60% loss of amplitude in the P30 response in each hemisphere.
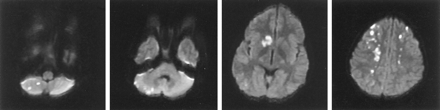
The patient awoke from anesthesia and was extubated. Motor examination findings were normal, yet the patient followed commands poorly and seemed confused. Later in the evening, the patient was incontinent of urine and failed to act appropriately with his mother. He was nearly mute and moderately combative. Electrolyte and oxygen saturation levels were normal. The following morning, cerebral MR imaging revealed diffuse patchy infarcts involving the caudate nuclei, deep white matter, and cerebellar hemisphere (Fig 2). A transthoracic echocardiogram with a bubble study demonstrated a right-to-left cardiac shunt consistent with a patent foramen ovale. A chest radiograph did not show signs of pulmonary arteriovenous fistulas. Over the next 3 days, the patient’s neurologic function returned to his baseline. Detailed neuropsychiatric testing was not performed.
Diffusion-weighted MR image shows multiple infarctions secondary to paradoxical emboli.
Discussion
A PFO is a persistent valvelike connection between the right and left atria that, in most individuals, is closed by fibrous adhesions between the septum primum and the septum secundum during the 1st month of life (2). Autopsy studies have demonstrated a prevalence of PFO of 17–35% (2). Many studies have linked PFO to paradoxical cerebral emboli (3–16).
When particulate emboli are intentionally injected into the arterial circulation to treat a lesion that contains arteriovenous shunts such as vascular tumors and vascular malformations, the likelihood of paradoxical embolization increases when a patient also has a PFO. In the case described here, the patient did not have a cardiac murmur, and his PFO manifested itself only during coughing. The circuit of the anesthesia machine induced 2.5 mmHg of positive end-expiratory pressure. This, combined with the particle size, (300–500 μm) may have been enough to promote the paradoxical events. In retrospect, neurophysiologic monitoring enabled the early detection of the events; however, we did not recognize their importance.
How has this event changed our practice? We have routinely used neurophysiologic monitoring with embolizations in patients under general anesthesia and will continue to do so. External carotid circulation procedures should never be considered low risk in view of the numerous, dangerous external carotid–to–internal carotid anastomoses that may not be apparent during superselective arteriography. The information provided by brainstem auditory evoked potentials and EEG and SSEP results, although not a substitute for a detailed neurologic examination, can help alert the physician to unexpected embolic events or changes in cerebral perfusion. We have always performed our particulate embolization procedures in awake, sedated patients when possible, and will continue to do so, to perform neurologic examinations throughout the procedure. Nevertheless, some individuals cannot tolerate an awake procedure. All patients undergoing nonemergent particulate embolization now undergo pretreatment echocardiography. Those who have a PFO or other atrial septal defect do not undergo embolization, or they are treated by using acrylic agents or larger particles that are less likely to migrate far along the venous system and back to the heart. We suspect that, in view of the relatively high prevalence of intracardiac shunts in the general population, this issue will present itself again soon.
References
- Received February 1, 2002.
- Accepted after revision March 4, 2002.
- Copyright © American Society of Neuroradiology