Abstract
BACKGROUND AND PURPOSE: In cases of metastatic disease of the spine, monitoring the response to medical therapy with plain radiography, bone scanning, and conventional spin-echo sequence MR imaging is unsatisfactory because of the insensitivity or nonspecific findings of these imaging modalities. The purpose of this study was to investigate signal intensity changes of bone marrow after therapy by using diffusion-weighted MR imaging to monitor the response to medical therapy in cases of metastatic disease of the spine.
METHODS: Twenty-four patients with metastatic disease of the spine were examined with MR imaging. Diffusion-weighted MR imaging and spin-echo MR imaging were performed in all patients before and after radiation therapy. Follow-up diffusion-weighted MR imaging and spin-echo MR imaging were performed for comparison purposes in nine cases at 1 month, in seven cases at 2 months, in seven cases at 3 months, and in three cases at 6 months after therapy. The diffusion-weighted MR imaging sequences were based on a steady-state free precession with a low b value (165 s/mm2) and a single shot stimulated echo-acquisition mode with a high b value (650 s/mm2). Apparent diffusion coefficient maps were obtained using two different b values incorporated in a diffusion-weighted single shot stimulated echo-acquisition mode sequence. Apparent diffusion coefficient maps were obtained in three cases. Signal intensity changes of the metastatic disease of the vertebral bone marrow before and after therapy on conventional spin-echo sequence and diffusion-weighted MR images were evaluated.
RESULTS: As shown by diffusion-weighted MR imaging, metastatic disease of the vertebral bone marrow included in our study before therapy was hyperintense to normal vertebral bodies. In 23 patients with clinical improvement, metastatic disease of the spine after therapy was hypointense relative to normal vertebral bodies on the follow-up diffusion-weighted MR images. In one patient with hepatocellular carcinoma, the clinical symptoms did not improve and follow-up bone scanning performed 6 months after therapy showed increased uptake. Persistent hyperintense bone marrow after therapy was also noted on diffusion-weighted MR images. Decreased signal intensity of the metastatic disease of the spine on diffusion-weighted MR images was observed >1 month after therapy.
CONCLUSION: Diffusion-weighted MR imaging shows that, with successful therapy, there is decreased signal intensity of metastatic disease of the vertebral bone marrow.
Bone metastases occur in 30% to 70% of patients with cancer and commonly involve the spine (1). In most cases of spinal metastases, radiation therapy, with or without systemic chemotherapy, is performed for palliative or curative intent (2). A reliable method of evaluating the response to treatment is required for optimal patient management, such as minimizing radiation dose or preventing recurrence. Although monitoring the response to therapy is very important, an accurate assessment of response to therapy is often difficult. With general monitoring, the response to therapy as revealed by plain radiography, bone scanning, and conventional spin-echo MR imaging is unsatisfactory because of the insensitivity or other limitations of these imaging modalities. Most physicians perform radionuclide bone scanning for monitoring the response in daily practice, but there are serious diagnostic pitfalls, such as scintigraphic flare (3), that can complicate the evaluation of the therapeutic response of spine metastasis. Although conventional spin-echo sequence MR imaging is very sensitive in the detection of pathologic lesions of bone marrow of the spine, specificity is often low because of nonspecific signal intensity changes.
Diffusion-weighted MR imaging is a method that reflects water mobility in vivo and has been used in imaging of the brain for early detection of regional cerebral ischemia (4), in discriminating between cytotoxic and vasogenic edema (5), and in the evaluation of cellularity in gliomas (6). It has not, however, been investigated for monitoring the response to therapy of metastasis to the spine. In our study, we evaluated the usefulness of diffusion-weighted MR imaging for monitoring the response to radiation therapy of metastatic disease of the spine.
Methods
Between January 1999 and December 2001, we prospectively examined 24 patients (17 female and seven male patients) with spinal metastases. Patient age ranged from 33 to 77 years, with an average age of 48 years. Primary neoplasms included invasive ductal carcinoma of the breast (n = 14), renal cell carcinoma (n = 2), adenocarcinoma of the stomach (n = 3), lung cancer (n = 2), squamous cell carcinoma of the uterine cervix (n = 1), cholangiocarcinoma of the liver (n = 1), and hepatocellular carcinoma (n = 1). Pathologic confirmation of the metastatic tumors was obtained in 11 cases with CT-guided needle biopsy, and clinical diagnosis was established in 13 cases by typical MR imaging findings, such as paravertebral soft-tissue mass, osteolytic change, and involvement of posterior elements of metastatic disease of the spine with primary neoplasms.
Thirty gray was delivered to the spine. Successful therapy response was evaluated based on relief of clinical symptoms, such as pain of involved sites, and based on decreased uptake shown on follow-up bone scans obtained >6 months after therapy.
In this study, MR imaging was performed with a 1.5-T imager with a spine array coil. For spin-echo sequences, axial and sagittal view T1-weighted (583/12 [TR/TE]) and turbo-T2–weighted (3800/128) images were obtained. The diffusion-weighted MR imaging sequence (21.6/5/18 [TR/TE/NEX]) was based on a steady-state free precession, in which the echo part of the steady-state free precession signal was used with a TR of 21.6 and a diffusion pulse length of 2 ms (matrix, 202 × 206; field of view, 228 × 260). The diffusion gradient strength was 24 mT/m, with a relatively low b value (165 s/mm2). The diffusion gradient was applied only in the readout direction based on the previous observation that no diffusion anisotropy was found in either the phase or section direction (7). Mainly sagittal view diffusion-weighted MR images were obtained, but additional axial views were obtained in cases of focal metastatic disease of the spine.
We also performed high-b-value diffusion-weighted MR imaging in three cases with spine metastasis. Primary tumors were lung cancer (two cases) and uterine cervical carcinoma (one case). To obtain diffusion-weighted MR images with a high b value (650 s/mm2) and apparent diffusion coefficient (ADC) maps, a diffusion-weighted single shot stimulated echo-acquisition mode sequence was obtained using the same 1.5-T MR imager. The diffusion gradient of this sequence was applied in the readout, phase, and section directions. The image parameters were 600/66/12, with a total imaging time of 6 min for a 60 × 128 matrix covering a rectangular 160 × 210 mm2. Whereas echo-planar imaging-based diffusion measurement shows image distortion and susceptibility artifact, which is problematic in imaging of the spine, the single shot stimulated echo-acquisition mode diffusion sequence is insensitive to such problems. ADC maps were calculated based on images without diffusion gradient (b = 0) and diffusion-weighted MR images (b = 650 s/mm2) by using the image processing software of the MR imager for automated online computations. Diffusion-weighted MR imaging and and spin-echo MR imaging were performed in all patients before and after therapy. Follow-up diffusion-weighted MR imaging and spin-echo MR imaging were performed to compare nine cases at 1 month, seven cases at 2 months, seven cases at 3 months, and three cases at 6 months after therapy.
Two experienced radiologists (W.M.B., S.J.L.) qualitively evaluated the signal intensity characteristics of metastatic disease of the spine by T1-, T2-, and diffusion-weighted MR imaging. They compared the findings obtained before therapy with those obtained after therapy.
Results
Twenty-three patients experienced improvement of pain and/or had decreased uptake shown on follow-up bone scans obtained 6 months after therapy. One patient with hepatocellular carcinoma, however, showed no improvement of clinical symptoms and had increased uptake in the second lumar body shown on follow-up bone scans. Another patient had recurrent metastasis in the left femur shown on follow-up bone scans obtained 6 months after therapy.
All metastasis to the spine before therapy was hypointense to normal bone marrow on T1-weighted spin-echo images and was hyperintense (18 cases) or hypointense (six cases) on T2-weighted spin-echo images. In 24 cases, metastasis to the spine before therapy was hyperintense to normal bone marrow on diffusion-weighted MR images (Figs 1 and 2A–C). Follow-up T1-weighted MR images revealed no interval change or slightly decreased change of signal intensity in metastatic disease of the spine. Slightly decreased signal intensities (13 cases), no interval change (10 cases), or high signal intensities (one case) associated with metastasis to the spine were seen on follow-up T2-weighted MR images. This indicated that spin-echo sequences are not helpful for evaluation of monitoring response to therapy (Figs 1 and 2D and E). However, for 23 patients who experienced clinical improvement, diffusion-weighted steady-state free precession MR images with a low b value (165 s/mm2) were hypointense to normal bone marrow >1 month after the end of therapy (Figs 1 and 2F). For the patient with hepatocellular carcinoma who showed no clinical improvement, persistent hyperintense bone marrow on follow-up diffusion MR images obtained 1 and 6 months after therapy was noted (Fig 3). On diffusion-weighted single shot stimulated echo-acquisition mode MR images with a high b value (650 s/mm2), metastatic disease of the vertebral bone marrow before therapy was slightly hyperintense in all three cases (Fig 4C). Follow-up diffusion-weighted MR images with a high b value showed hypointense marrow in three cases (Fig 4G). Normal bone marrow, on the other hand, showed little signal attenuation, even with a high b value of 650 s/mm2, on initial and follow-up studies. On calculated ADC maps, the metastatic disease of the vertebral bone marrow was hyperintense to normal bone marrow in all three cases on initial and follow-up studies (Fig 4D and H). ADC value of metastatic disease of the vertebral bone marrow was (0.78 ± 0.03) × 10−3 mm2/s on initial study, and the value increased to (1.22 ± 0.02) × 10−3 mm2/s on the follow-up study. Normal bone marrow showed a minimal ADC value of (0.33 ± 0.03) × 10−3 mm2/s on initial and follow-up studies.
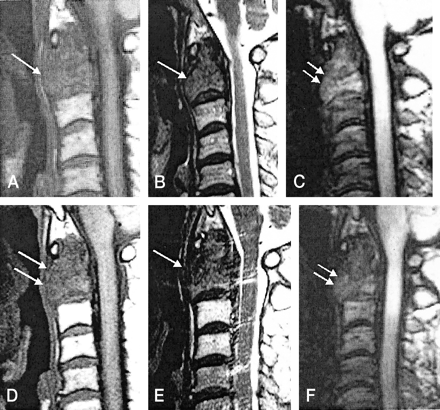
C2 metastasis in a 60-year-old male patient with renal cell carcinoma.
A, T1-weighted MR image (583/12 [TR/TE]) obtained before radiation therapy shows low signal intensity in the metastatic lesion (arrow).
B, T2-weighted MR image (3800/128) obtained before radiation therapy shows low signal intensity in the metastatic lesion (arrow).
C, Diffusion-weighted MR image (TR, 21.6; diffusion pulse length, 2 ms) obtained before radiation therapy shows slight hyperintensity (arrows) relative to normal vertebral bone marrow.
D, Follow-up T1-weighted MR image reveals persistent hypointensity (arrows) 1 month after therapy.
E, Follow-up T2-weighted MR image reveals persistent hypointensity (arrow) 1 month after therapy.
F, Follow-up diffusion-weighted MR image obtained 1 month after therapy shows iso- to hypointense signal (arrows). In a case of metastasis to the spine with clinical improvement, diffusion-weighted MR image shows decreased signal intensity change whereas conventional spin-echo T1- or T2-weighted MR images are not completely conclusive for monitoring the response.
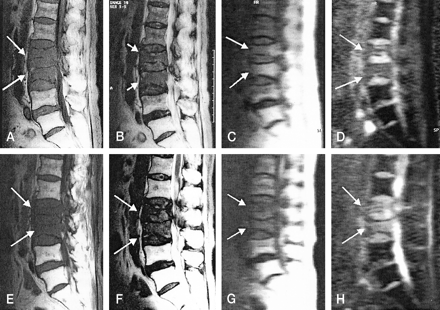
T2 metastasis in a 36-year-old female patient with invasive ductal carcinoma of the breast.
A, T1-weighted MR image (583/12) obtained before radiation therapy shows focal hypointense metastatic lesion (arrow) in right posterior portion of the vertebral body.
B, T2-weighted MR image (3800/128) obtained before radiation therapy shows focal hypointense metastatic lesion (arrow) in right posterior portion of the vertebral body.
C, Diffusion-weighted MR image (TR, 21.6; diffusion pulse length, 2 ms) obtained before radiation therapy reveals hyperintensity (arrows) relative to normal bone marrow.
D, Follow-up T1-weighted MR image shows persistent hypointense signal (arrow).
E, T2-weighted MR image reveals hyperintense signal (arrow).
F, Diffusion-weighted MR image shows hypointensity (arrow) relative to normal bone marrow 2 months after therapy.
G, Diffusion-weighted MR image obtained 3 months after therapy shows more hypointense change (arrows). Longer follow-up diffusion-weighted MR image shows more hypointense change in metastasis to the spine with clinical improvement.
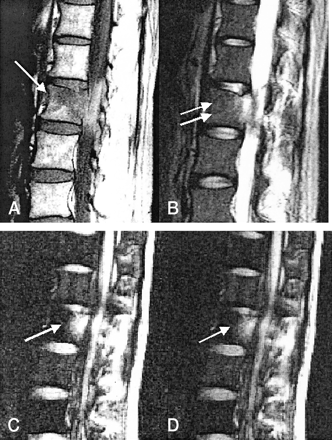
L2 metastasis with no response to therapy in a 37-year-old female patient with hepatocellular carcinoma.
A, T1-weighted MR image (583/12) obtained before radiation therapy shows hypointense change (arrow).
B, T2-weighted MR image (3800/128) obtained before radiation therapy shows high signal intensity (arrows).
C, Diffusion-weighted MR image obtained before radiation therapy shows hyperintense metastatic tumor (arrow).
D, Diffusion-weighted MR image obtained after radiation therapy shows hyperintense metastatic tumor (arrow). This patient experienced persistent back pain and had no decreased uptake shown on a bone scan obtained 6 months after therapy. With metastasis to the spine without clinical improvement, persistent hyperintense bone marrow on follow-up diffusion-weighted MR images obtained after therapy is noted.
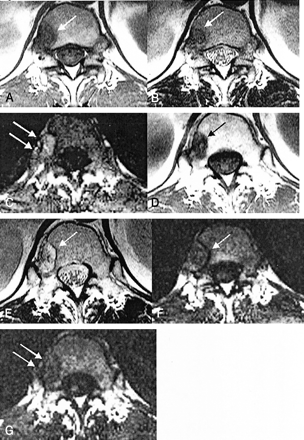
L2 and L3 metastasis in 70-year-old female patient with uterine cervix carcinoma.
A, T1-weighted MR image (583/12) obtained before radiation therapy shows low signal intensity in metastatic diseases (arrows). Hyperintense bone marrow caused by previous radiation therapy in the pelvic cavity can be seen.
B, T2-weighted MR image (3800/128) obtained before radiation therapy shows low signal intensity in metastatic diseases (arrows).
C, Diffusion-weighted MR image with high b value (650 s/mm2), obtained before therapy, shows slight high signal intensity in the metastatic disease (arrows).
D, Metastatic disease before therapy reveals high signal intensity on ADC maps (arrows). ADC value is (0.82 ± 0.03) × 10−3 mm2/s.
E, T1-weighted MR image (583/12) obtained after radiation therapy shows low signal intensity in metastatic diseases (arrows).
F, T2-weighted MR image (3800/128) obtained after radiation therapy shows low signal intensity in metastatic diseases (arrows).
G, Diffusion-weighted MR image with high b value (650 s/mm2), obtained after therapy, shows hypointensity in metastatic diseases (arrows).
H, ADC maps obtained after therapy reveal high signal intensity (arrows), and the ADC value ([1.33 ± 0.09] × 10−3 mm2/s) is increased. Normal bone marrow on ADC maps appear dark before therapy (as shown in D) and after therapy (as can be seen in this image) because of minimal diffusion of normal bone marrow. On ADC maps, ADC values after therapy are increased compared with those before therapy because of an increased extracellular volume fraction caused by necrosis of the tumor cells and bone marrow elements.
Discussion
The spine is a common site of metastatic spread, which causes major clinical problems. Metastatic disease mostly affects the vertebral bodies, and pathologic compression fractures are common. With spin-echo MR imaging, it is often difficult to discriminate whether the cause of acute vertebral compression fracture is osteoporosis or metastasis (8, 9). Although several reports have shown the radiologic findings of benign and malignant causes of spinal collapse, discrimination between them is often difficult with plain radiography, CT, and conventional spin-echo MR imaging.
Baur et al (10) reported that diffusion-weighted MR imaging with a steady-state free precession sequence of the vertebral bone marrow provides excellent distinction between malignant and benign compression fractures. They noted that diffusion-weighted MR imaging shows pathologic compression fractures of the spine to be hyperintense to adjacent normal vertebral bodies, but it shows benign compression fractures to be hypo- to isointense to normal vertebral bodies. A possible explanation for the results in cases of malignant fracture is that reduction of the extracellular volume in densely packed tumorous tissue might lead to a decrease in the ADC and increased signal intensity. Castillo et al (11) reported that with diffusion-weighted MR imaging in cases of metastatic disease, variable signal intensities (hypointense, hyperintense, and mixed) were detected. They concluded that diffusion-weighted MR imaging of the spine showed no advantage in the detection and characterization of vertebral metastases as compared with unenhanced T1-weighted MR imaging. However, they reported that metastatic disease of the spine with primary prostate tumor was hypointense on diffusion-weighted MR images. Although that particular metastatic disease was not included in our study, diffusion-weighted MR images revealed hypointensity (two patients). Two patients with prostate metastasis were not included in our study because follow-up studies were not performed. The metastatic diseases to the spine included in our study were hyperintense compared with adjacent normal bone marrow of the spine on both low-b-value and high-b-value diffusion-weighted MR images.
In our study, the diffusion-weighted MR imaging sequence with a low b value was based on a steady-state free precession sequence. This sequence is known to have strong diffusion-weighting effect, even with a low b value, because of the contribution of stimulated and higher order echoes coherent to the primary spin echo. However, the strong dependence of diffusion-weighted steady-state free precession signal on tissue relaxation times (T1 and T2) and imaging parameters make it difficult to estimate a global b value and thus to calculate the ADC without knowledge of the accurate relaxation times and imaging parameters, such as flip angle (12). Therefore, to obtain an ADC map, the diffusion-weighted single shot stimulated echo-acquisition mode sequence was used with a section-selective spin-echo preparation that replaces the leading 90 RF pulse of a conventional single shot stimulated echo-acquisition mode sequence. This spin-echo period symmetrically encompasses the diffusion gradients as a conventional Stejskal-Tanner scheme. In this scheme, an ADC value can be easily calculated with a well-defined b value.
With most metastatic disease of the spine, a common purpose of therapy is pain relief and preservation of skeletal integrity. Pain relief and bone stabilization are the methods by which the medical goals of patient comfort and independence are achieved. Symptomatic relief is usually satisfactorily achieved with radiation therapy and chemotherapy. In general, the response may be evaluated by clinical symptoms and/or plain radiography, whole-body bone scanning, and conventional spin-echo MR imaging. Currently available imaging modalities for response to therapy are nonspecific or limited. Roentgenographic evidence of healing may not be visible for 6 months or more and will appear as static disease on plain radiographs (13).
Whole-body bone scanning has been widely used to monitor response to therapy. One limitation of this technique is that it measures only metabolic activity and does not evaluate the structural integrity of the bone. Biologic control of the tumor does not always translate into mechanical restoration of the skeleton. Therefore, bone scan findings must be evaluated in parallel with findings of plain radiography, CT, or both. Moreover, a serious pitfall in using bone scan in the evaluation of therapy response is scintigraphic flare. Scintigraphic flare, such as apparent deterioration caused by an intense osteoblastic response reflecting healing in association with response to therapy, has been well described in several articles (3, 14–16). Vogel et al (17) noted that changes in bone scintigraphy that mimic progressive disease early in the course of treatment of patients with breast cancer that is metastatic to bone may represent scintigraphic flare associated with response. Therefore, they urged clinicians to be aware of the phenomenon of scintigraphic flare to avoid premature discontinuation of a potentially beneficial treatment. Bone scan flare may seriously complicate evaluation of the therapeutic response of spine metastasis. Levenson et al (18) reported the usefulness of radionuclide bone scans and bone radiographs in evaluating tumor response to therapy. They concluded that although bone scans are useful for monitoring tumor response, radionuclide bone scans are not accurate enough to be used as the sole modality in evaluating tumor response to therapy because of the lack of sensitivity to response and flare phenomenon in some patients. Because scintigraphic flare is observed approximately 3 months after the initiation of therapy for bone metastasis, it may not be possible to evaluate response to therapy accurately until ≥6 months after therapy (13, 19).
Conventional spin-echo MR imaging was performed before and after therapy, which may not be helpful for monitoring the response to therapy of metastatic disease of the spine. In our studies, most metastasis to the spine before therapy showed hypointensities on T1-weighted MR images and low to high signal intensity on T2-weighted MR images, but follow-up MR images obtained ≥1 month after therapy showed persistent abnormal signal intensities, such as hypointensity on T1-weighted MR images and variable signal intensities on T2-weighted MR images.
In this study, decreased signal intensity of the metastatic disease of the spine on both low-b-value and high-b-value diffusion-weighted MR images began to be observed >1 month after therapy. Our results also showed that normal bone marrow has low diffusion-related signal change between b = 0 and b = 650 s/mm2 before and after therapy. This suggests that normal bone marrow has a low ADC value. The minimal diffusion of normal bone marrow obtained in our ADC measurement is well in agreement with that of a previous report (20). Because of minimal diffusion of normal bone marrow, metastatic disease of the spine appeared hyperintense compared with normal bone marrow on the ADC maps obtained before and after therapy. However, brightness of hyperintensity, which is proportional to ADC value, increased after therapy with statistical significance (P < .05).
The hyperintense signal of metastatic disease of the spine on diffusion-weighted MR images has been previously explained to result from decreasing extracellular spaces caused by a compact accumulation of tumor cells (10). In this study, on follow-up diffusion-weighted MR images obtained after therapy, decreased signal intensities were shown in all cases. A possible explanation for hypointense metastatases of the spine after therapy is that necrosis of the tumor cells and bone marrow elements leads to an increased extracellular volume fraction. Our quantitative ADC measurement seems to support these explanations.
Conclusion
Our investigation shows that in cases of metastatic disease of the spine, diffusion-weighted MR imaging can be used to monitor the response to therapy whereas conventional spin-echo T1- or T2-weighted MR imaging may not be conclusive for monitoring that response. Metastatic disease of vertebral bone marrow before therapy was hyperintense relative to normal spinal bodies on diffusion-weighted MR images. With clinical improvement, metastatic disease of the bone marrow after therapy was hypointense relative to normal vertebral bodies. These findings were also confirmed by measuring the quantitative ADC values, which increased after therapy.
Footnotes
Supported by a grant from Hankok Medical Science Foundation.
Presented in part at the 38th Annual Meeting of the American Society of Neuroradiology, Atlanta, 2000.
References
- Received July 30, 2001.
- Accepted after revision February 19, 2002.
- Copyright © American Society of Neuroradiology