Abstract
BACKGROUND AND PURPOSE: We herein present our experience in diagnosing and treating 13 children with vein of Galen aneurysmal malformations (VGAM), with an emphasis on possible prognostic indicators, endovascular strategies, factors affecting treatment during the neonatal period, and long-term follow-up. With this review, we hope to identify those factors that have the most significant prognostic value in determining long-term outcomes in children with VGAM.
METHODS: We retrospectively reviewed the radiology studies, hospital charts, and outpatient clinic chart notes (when applicable) of 13 children evaluated and treated for VGAM at a single tertiary care pediatric hospital. Clinical presentation, diagnostic methods, treatment strategies, and outcome were documented for each child. The present neurologic status and level of function of each patient was determined by review of the outpatient charts and direct contact with the clinicians who were conducting the follow-up. Outcome was graded on a 5-point scale, ranging from 0 (death) to 4 (normal), taking into account only neurologic and developmental characteristics.
RESULTS: Eight of 13 patients presented as neonates with congestive heart failure. The other five patients ranged in age from 4 months to 13 years at the time of presentation. The five patients presenting outside of the neonatal period achieved normal or near-normal outcomes. Two of the eight patients presenting during the neonatal period achieved normal or near-normal outcomes, one experienced significant impairment, and the other five died. We were unable to identify significant differences in outcome on the basis of differences in treatment strategies.
CONCLUSION: Our experience confirms that children with VGAM presenting during the neonatal period have a generally much worse prognosis than do those presenting later in childhood. Complicating factors in the management and treatment of these children are discussed in light of their impact on outcome.
Vein of Galen aneurysmal malformations (VGAM) are rare congenital vascular malformations characterized by shunting of arterial flow into an enlarged cerebral vein dorsal to the tectum. Most of these malformations present in early childhood, often causing congestive heart failure in the neonate. With the advent of endovascular neurointerventional techniques, the prospects for successful treatment of these lesions, once dismal, are now much improved. Several treatment strategies have been advocated in the medical literature, varying in recommended avenue of approach (arterial or venous), embolization material (coils or acrylics), timing of treatment, and management of associated conditions such as hydrocephalus. We herein present our experience in diagnosing and treating 13 children with VGAM, with an emphasis on possible prognostic indicators, endovascular strategies, factors affecting treatment during the neonatal period, and long-term follow-up. By this review, we hope to identify those factors that have the most significant prognostic value in determining long-term outcomes for children with VGAM.
Methods
During the 14-year period from 1987 to 2001, we diagnosed and treated 13 cases of VGAM in 13 children. All 13 children underwent diagnostic angiography and some form of catheter embolization. Ten underwent preoperative CT of the head, and six underwent preoperative MR imaging with MR angiography. Lesions were categorized as choroidal, mural, or mixed on the basis of angioarchitecture revealed by cerebral angiography. The radiology studies, hospital charts, and outpatient clinic chart notes (when applicable) of each patient were retrospectively reviewed. The present neurologic status and level of function of each patient were determined by review of the outpatient charts and direct contact with the clinicians who were conducting the follow-up. Outcome was graded on a 5-point scale, ranging from 0 (death) to 4 (normal), taking into account only neurologic and developmental characteristics. A score of 1 indicates severe neurologic impairment, requiring intensive supportive care and medical management. A score of 2 indicates the presence of moderate neurologic impairment affecting activities of daily living and requiring significant support in educational and social interactions but a lesser degree of daily medical management. A score of 3 reflects a milder degree of impairment, resulting in some need for social and educational support, with medical management only on occasion.
Results
The results are summarized in Tables 1 and 2.
Clinical presentation and outcomes of 13 children with vein of Galenaneurysmal malformation
Fistula classification, treatment variables, and outcomes for 13 children with vein of Galen aneurysmal malformation
Effect of Clinical Presentation on Outcome
Eight of the children had their condition diagnosed when they were neonates, four between 4 and 19 months of age, and one at 13 years of age (Table 1). All eight of the patients presenting during the neonatal period were experiencing some degree of heart failure at the time of presentation. Of the eight, two were essentially normal during follow-up; one of the two had experienced only mild cardiac failure at the time of presentation. Of the remaining six, four died shortly after embolization, one died 18 months after embolization, and one had marked neurologic deficits 24 months after embolization. One child died as a consequence of intracranial hemorrhage associated with embolization, and three children died as a result of multiple organ failure shortly after embolization. One child died as a consequence of progressive cerebral volume loss and associated neurologic deterioration occurring over 18 months.
The five patients presenting outside of the neonatal period achieved normal or near-normal outcomes. In all five cases, congestive heart failure was mild or absent at presentation. No continued effect of the malformation on cardiac function was observed in these five patients, even in those cases without complete angiographic exclusion of the malformation. The three patients presenting with hydrocephalus and no congestive heart failure had achieved good outcomes at the time of this writing (grades 3 and 4), with management of hydrocephalus being the major ongoing clinical concern. Thus, overall, 54% of the patients achieved good outcomes (25% of the neonates and 100% of the non-neonates).
Effect of Angioarchitecture on Outcome
Five of the 12 malformations were classified as choroidal, seven as mural, and one as mixed, with both choroidal and mural components identifiable (Table 2). Two of the five children with choroidal lesions died, and one had significant impairment; the remaining two achieved good outcomes. Three of the seven children with mural lesions died shortly after treatment; the other four achieved normal or near-normal development after treatment. The only patient with mixed lesions in this group continued to thrive and develop normally at the time of this writing.
Effect of Treatment Strategies on Outcome
All 13 patients underwent transcatheter embolization. An arterial approach only was used in six children, a venous approach only was used in one child, and both arterial and venous approaches to the malformation were used in six children (Table 2). A transtorcular approach was used in two children: one achieved a very good outcome, and one died. In seven cases, coils were placed on both the arterial and venous sides of the malformation; five of those patients did poorly, and two achieved good outcomes. Five of the six children with embolization of only arterial feeders achieved normal or near-normal outcomes; the fifth child died during the peri-procedural period. For two children, liquid acrylics were used in addition to pushable coils as the embolic material.
Six of the children required ventricular shunt surgery. Four achieved good outcomes, and two did poorly. There was no appreciable improvement in outcome from the beginning to the end of the 15-year period encompassed by this review (Table 1). There was no significant change in the personnel comprising the neurosurgical and neuroradiologic team managing these cases during that period.
Illustrative Cases
Patient 6
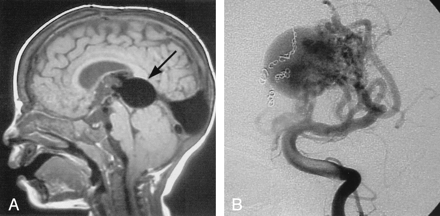
This child presented at 19 months of age with an increasing head circumference, engorged scalp veins, and mild congestive heart failure. CT and MR imaging revealed a VGAM (Fig 1). Subsequent angiography showed that the lesion had multiple direct fistulae to the wall of the dilated vein and a complex arterial maze at the anterior aspect of the vein. The fistulae were embolized with multiple coils via an arterial approach in two sessions. Radiosurgery was then used, with 2000 cGy delivered in a single treatment to the site of residual arteriovenous shunting, as shown on MR images. Follow-up with MR angiography showed a progressive decrease in the size and complexity of the arterial maze during a 4-year period, and the child was developing normally and was not receiving any cardiac medications.
Images from the case of a 19-month-old patient with mild hydrocephalus and engorged scalp veins.
A, Sagittal view T1-weighted MR image shows the markedly enlarged median prosencephalic vein of Markowski, characteristic of VGAM (arrow). Arterial feeders can be seen along the anterior wall of the vein.
B, The complex arterial maze (arrows) is well seen on this conventional angiogram obtained with injection of the left vertebral artery. Coils can be seen along the right side of the varix, occluding several arterial feeders.
Patient 7
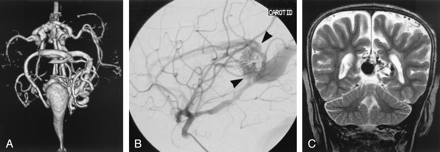
This child was one of triplets, with a birth weight of <1500 g. Ultrasonography of the head was performed shortly after birth as part of a screening process for high risk neonates; it revealed a VGAM (Fig 2). The patient had mild symptoms of congestive heart failure, responsive to medical management. Embolization was performed at 3 months of age because of worsening cardiac function, with coils placed into several arterial feeders from a venous approach. A second embolization was performed at 6 months of age, with more arterial feeders occluded from an arterial approach. The patient developed appropriately from that time, with no requirement for cardiac medication and no hydrocephalus. Follow-up MR imaging showed continued flow into the dilated vein from several remaining arterial feeders. Further treatment was not pursued in the absence of signs or symptoms.
Images from the case of a 3-month-old triplet with worsening congestive heart failure.
A, Volume-rendered MR angiogram shows, from an inferior prospective, the arterial feeders supplying the lesion from the left side (arrows).
B, Carotid injection during arterial embolization procedure performed when the patient was 6 months old shows residual flow to the lesion from pericallosal and posterior choroidal arteries (arrowheads).
C, Coronal view fast spin-echo T2-weighted MR image obtained 6 years after embolization shows continued patency of the varix and mild enlargement of extra-axial fluid spaces.
Patient 10
This child presented as a neonate with a markedly enlarged head circumference. VGAM had been identified in utero, and delivery by C-section was performed at 36 weeks. MR imaging of the brain showed a markedly enlarged vein draining the malformation that narrowed just proximal to the torcula. Angiography showed several simple fistulae to the wall of the dilated vein, without a complex arterial maze. Pushable coils were placed into several feeding arteries from an arterial approach, resulting in a marked decrease of flow through the malformation. During occlusion of a remaining pericallosal feeder, a sudden increase in heart rate and blood pressure was recognized. Withdrawal of the microcatheter and digital subtraction angiography showed extravasation of contrast material from the artery proximal to the site of occlusion. The site of extravasation was successfully occluded with coils, but subsequent imaging showed extensive subarachnoid hemorrhage and diffusely decreased parenchymal attenuation. The patient was declared brain dead and died within 24 hours.
Discussion
Understanding of the VGAM has advanced greatly during the past 2 decades. By analyzing the vascular anatomy of VGAM at angiography and correlating the findings with the known embryologic development of the cerebral vasculature, Raybaud et al (1) concluded that the malformation develops between the 6th and 11th weeks of gestation, after development of the circle of Willis. It is thought to result from the development of an arteriovenous connection between primitive choroidal vessels and the median prosencephalic vein of Markowski. The abnormal flow through the connection retards the normal involution of this embryonic vein and thus prevents the development of the vein of Galen. The shunt is maintained through the remainder of brain development, with the persistent median vein draining into the sagittal sinus, often via a persistent falcine vein, with absence of the straight sinus. Other venous anomalies are commonly present in association with VGAM, including anomalous dural sinuses and sinus stenoses (1–3). Some authors think that it is the presence of these stenoses that cause the dilatation of the vein (2, 4). These stenoses may be acquired as a response of the venous endothelium to the rapid and turbulent flow caused by the shunt (5). In our series, stenoses were identified in several cases, but venous enlargement was apparent in all. Alternative pathways of venous drainage typically develop to accommodate the markedly increased blood volume associated with the shunt (1, 4, 6). The adequacy of these alternative pathways may be one of the most important factors in determining long-term prognosis.
Because of their superficial similarity, arteriovenous malformations resulting in variceal enlargement of the true vein of Galen have sometimes been categorized in association with VGAM. Termed vein of Galen aneurysmal dilatations, these lesions are characterized by drainage of an arteriovenous malformation or dural fistula into the true vein of Galen. The dilated vein in these cases drains brain parenchyma in addition to the malformation, as opposed to the persistent embryonic vein in the true VGAM that drains only the malformation (4). All 13 cases presented herein were true VGAM.
There have been several proposed classifications of VGAM, based on complexity, type of supplying arteries, location of fistula, and degree of venous ectasia (2, 4, 7). In the radiologic literature, one favored classification divides VGAM into choroidal or mural subtypes. The choroidal type is characterized by multiple feeders from the choroidal arteries and other deep midbrain arteries that converge on a fistula site at the anterior aspect of the median vein. The appearance of these malformations has been described as a complex arterial maze and may mimic a true AVM. Mural lesions, by contrast, are characterized by a fistula or fistulae in the wall of the median prosencephalic vein, often located laterally. They typically have fewer feeding arteries, do not have a complex arterial maze, and are said to present with lesser degrees of heart failure (2, 4, 8). Using this scheme, we classified seven of 13 lesions as mural, five as choroidal, and one as having both mural components and a more complex nidus anteriorly. Of note, five of the cases classified as mural had at least some degree of congestive heart failure. We were not able to distinguish differences in outcome between the two major groups and have not found use of this classification to be of significant benefit in clinical evaluation and treatment.
Before the development of catheter embolization techniques, treatment of VGAM was limited. Surgical treatment of these malformations was often dissatisfying, with a reported mortality rate of almost 90% after surgery in neonates. Endovascular techniques have markedly increased the odds for successful treatment of these lesions. Although some continue to advocate a surgical approach to these lesions (9, 10), most authors do not support this conclusion (2, 4–7, 11–14). Initially, a transtorcular embolization of the dilated vein itself was proposed as the ideal approach (6, 15–18); more recent reports have emphasized the benefits of a primary arterial occlusion (2, 4, 19–21). Similar to the experience of other authors, we have found greater success in those cases in which embolization was limited to the fistula point and arterial feeders of the malformation. Even when forced to use a venous approach to the lesion, we were able to embolize arterial feeders through retrograde selection in most cases. Proponents of primary venous occlusion have pointed out that a graded obliteration of the fistula is possible by this method, with measurement of venous pressure during embolization (15–17). Our experience suggests that incomplete or limited obliteration may be ideal (see below); however, partial venous occlusion could pose inherent risks. The possibility of hemolysis caused by continued flow through an incompletely obstructing venous coil mass must be considered. Postprocedural disseminated intravascular coagulopathy has also been reported in association with this technique (22, 23). In our cases, vein occlusion was performed only when further treatment was necessitated by continued cardiac compromise and further arterial occlusion was no longer possible.
Several authors strongly advocate the use of liquid acrylic embolic agents for the treatment of VGAM (2, 4, 21). One benefit of these agents is the ability to inject them through very small catheters that may be more capable of negotiating the tortuous anatomy inherent in VGAM. Acrylics provide permanent occlusion, minimizing the risk of recanalization. In experienced hands, their use can dramatically reduce procedure time. Until recently, there have been limitations on the use of these agents in the United States (24). There is still a limited indication for their use in other pediatric endovascular treatments. We have found that pushable coils provide the same degree of permanence of occlusion as do acrylics, especially when used in combination with “liquid coils” (Boston Scientific, Fremont, CA). Newer 4F guide catheters will allow the passage of most microcatheters, providing the ability to acquire ideal distal microcatheter position for coil placement. The use of coils allows a greater degree of control over the exact location of occlusion and thus decreases the risk of distal migration of embolic material. In addition, if distal migration of coils occurs, the coils can be retrieved, unlike casts of acrylic.
We used radiosurgery as a treatment in one of our patients; radiosurgery is a treatment strategy that has been strongly discouraged by others (25). Because of the continued abnormal hydrodynamics caused by the malformation, progressive brain injury can occur during the 2- to 3-year period necessary for the effects of radiation treatment to manifest. We think that our case is the rare exception to this principle. The embolization of mural feeders to the shunt reduced flow enough to eliminate the risk of progressive brain injury, but the presence of the residual arterial maze continued to pose a risk and could potentially recruit more collateral flow if untreated. We have also used radiosurgery with success in an adult patient with a choroidal VGAM (not included in this series), under similar unusual circumstances (26). In most cases, the option of such gradual treatment is not feasible.
Our group of patients is skewed toward those presenting during the neonatal period, comprising 62% of our cases. One would presume that the increased use of routine prenatal sonography would result in a greater number of prenatal diagnoses, but our experience does not support that conclusion. Only four cases in the 13 children were diagnosed prenatally, and we identified no trend toward earlier diagnoses in the more recent cases. The relatively poor outcomes for those children in our series who received their diagnosis and treatment while they were neonates mirrored, to some extent, the outcomes reported in the literature (2, 8, 27). Series with optimal outcomes in this age group have typically applied clinical criteria (see below) to determine eligibility for treatment (2). Generally speaking, these children are sicker than those with delayed presentation. The severity of cardiac dysfunction reflects a larger shunt, and the risks of perfusion pressure breakthrough with embolization are therefore greater. As a consequence of their uncontrollable congestive heart failure, seven of the eight neonates in our series were treated within the first week after diagnosis. Six of these infants fared very poorly. The one good outcome achieved in this group occurred in a child who underwent embolization twice during the first week of life, with coils and acrylic, with both venous and arterial occlusion. Although this was the only neonate in this group who was treated with acrylic embolization, it is unlikely that this played a significant role in the outcome; acrylic was used in a limited manner during the first embolization procedure, and a second procedure was required within a week because of limited effect. Another factor that may influence outcome in this group is the added technical difficulties posed by treating neonates. The femoral artery of a neonate is small and will not accommodate catheters larger than 4F, in our experience. If treatment is necessary during the first week of life, the umbilical artery is a more satisfactory site of access, but it may not be accessible at the time of embolization. This catheter size limitation may restrict the ability to gain ideal access to the AVF site and may limit the type of embolic material available.
When a VGAM is diagnosed by using prenatal ultrasonography, early delivery by means of caesarian section or induction of labor may be proposed (28). Early delivery may be necessitated by fetal demise, but the intent should be to enable more effective cardiac evaluation and management rather than more prompt embolization (29, 30). The low systemic resistance of the fetus in utero can decrease the flow through the fistula and minimize cardiac decompensation, but the sudden increase in systemic vascular resistance encountered at the time of delivery will result in a much greater diversion of flow through the malformation (27). Evidence of progressive cardiac dysfunction in utero is a grave sign, indicating a high flow lesion that may not respond to therapy (30). Five of the eight neonates in our group were premature, three as a consequence of prenatal diagnosis of VGAM. One of these five had treatment delayed for 3 months and eventually achieved a good outcome. The other four were treated within the first week of life; all four experienced poor outcomes, including the three who were purposely delivered early.
Although the drainage of the VGAM is initially segregated from cerebral venous drainage, they intersect at the level of the torcula. The high flow and pressures resulting from the fistula may cause venous stenoses. Stenosis or occlusion at the jugular bulb is a known sequela of VGAM in many cases. This can be tolerated if alternate cerebral venous channels are developed enough to accommodate the excessive venous flow. Even in the absence of jugular stenosis, the presence of a VGAM may stimulate recruitment of cerebral venous structures to handle the large volumes of blood shifted into the venous system after delivery. If the cerebral venous system is not mature enough to tolerate these volumes, there will be a resultant transmission of pressure back into the parenchyma and diminished arterial-to-venous gradient. It is reasonable to postulate that the presence of a VGAM may retard or alter the development of normal intracranial venous drainage in utero. This lack of venous maturity is likely accentuated in the premature infant. The cerebral venous insufficiency may prevent a good outcome in the face of uncomplicated exclusion of the malformation. We suspect that this physiology may have been present in five of the six neonates in our group who had poor outcomes. The sixth child experienced multiple medical complications as a consequence of prematurity that confused the clinical picture. Of the seven patients who achieved good outcomes, five did not have complete angiographic exclusion of the malformation, suggesting that an ideal angiographic result may not be an appropriate goal in the treatment of these children. Two of these five patients did appear to have thrombosed malformations, as revealed by subsequent imaging studies. Avoiding complete closure of the malformation may be even more important in neonatal patients, because their immature cerebral vascular bed may have difficulty tolerating the dramatic changes in blood volume and pressure associated with closure of the shunt. Avoiding this perfusion pressure breakthrough is the logic behind staged embolization of many vascular malformations, including VGAM.
We observed intraventricular hemorrhage after embolization in three of our patients; two of them were premature neonates. In the neonates, the bleeding was presumed to arise from immature vessels in the choroid plexus or germinal matrix that could not tolerate the significant increase in pressure and volume associated with occlusion. In an older infant, intraventricular hemorrhage extended from a hemorrhagic infarction, presumed to be venous. In one child (see above), subarachnoid hemorrhage developed during the embolization procedure itself, either from injury to the vessel during catheter placement, perfusion pressure breakthrough, or a combination of both factors. Intracranial hemorrhage was observed in another case as a consequence of shunt placement. In none of our cases did we observe intracranial hemorrhage before treatment, but this has been reported as an infrequent complication of VGAM (31). Some authors have suggested that the presence of a venous stenosis is a risk factor for the development of intracranial hemorrhage in association with VGAM (31).
The cause and management of hydrocephalus in children with VGAM is not entirely clear. Although it may be reasonable to assume that the dilated vein causes some direct compression of the cerebral aqueduct, sagittal MR imaging of several of our patients did not show obstruction at the level of the aqueduct. The absence of frank obstruction does not exclude the presence of an elevated pressure gradient across the aqueduct, however. We observed caudal displacement of the cerebellar tonsils in one infant in our series but did not have other indications that CSF obstruction at the foramen magnum contributed to the development of hydrocephalus in these children. More likely, elevated sinus pressures and cerebral blood volumes well beyond the capacity of the cerebral vascular system hampered the normal migration of CSF into the venous sinuses (15, 32, 33). Persistent venous insufficiency after malformation closure could explain the hydrocephalus, progressive atrophy, and parenchymal calcification observed in some patients with VGAM. It has been reported that this “brain melting” phenomenon can be accelerated by shunt placement (2). Lasjaunias (2) and Zerah et al (32) proposed that CSF diversion results in a reversal of flow in the medullary veins from a cerebro-fugal direction to a cerebro-petal one. This, in turn, causes diminished parenchymal perfusion and progressive deterioration. Ventricular shunt placement in children with VGAM is associated with a high risk of complication, regardless of whether the malformation has been embolized (34). It has been proposed that the engorged superficial veins associated with VGAM pose a considerable risk of hemorrhage with shunt placement. Some have even recommended that shunts be placed before embolization, presuming that venous engorgement increases as a result of decreasing flow through the shunt (6, 35). It is our practice to avoid ventricular shunt surgery until after all embolization procedures have been performed, and then only if there is documented evidence of progressive hydrocephalus. As recommended by others (34), we think that a frontal trajectory is likely more safe, avoiding enlarged veins frequently present over the parietal lobes.
Six of the patients in our series underwent MR imaging with MR angiography as part of their diagnostic evaluation before treatment. We have found MR angiography to be an invaluable tool in the analysis of these lesions before embolization. By depicting all the arterial feeders in a single image, the complexity of the lesion and anatomy of the fistula point can be elegantly shown. Data can be presented in a volume-rendered format, allowing many more perspectives than are possible by using conventional angiography. CT angiography may be of value in pretreatment analysis, providing a simultaneous depiction of venous and arterial anatomy (36). However, use of iodinated contrast material should be limited in these children, because the shunt may be a cause of renal dysfunction (2). Venous anatomy is well shown by MR venography, in our experience. We also use MR imaging with MR angiography as the primary follow-up tool for these patients. We have found that MR imaging cannot adequately evaluate the conditions of patients who have undergone placement of Gianturco coils into the venous pouch because of the extensive artifact caused by the coils. Because they are not paramagnetic, the smaller pushable coils that we use for arterial occlusion cause no image degradation when using MR imaging or MR angiography.
Several authors have proposed that a clinical evaluation scale be used to identify those children who will not respond well to treatment, so as to avoid fruitless procedures and prolonged morbidity. Findings that have been proposed as contraindications to treatment include prenatal cardiomegaly and cerebral injury evident at birth (30,37–39). The protocol used by the group at Bicêtre, who have treated >100 children, grades the function of major organ systems that are frequently impaired in these children. The same scale is used to determine optimal timing of embolization (2). We did not have sufficient clinical data to use such a grading system in a retrospective manner for this group of patients. However, it is fair to say that at least three of the six neonates in our group who experienced poor outcomes would not have met the threshold for early treatment by using such criteria, based on prenatal cardiomegaly and imaging evidence of brain damage. During past several years, we have encountered two other neonates with VGAM who did not proceed to treatment. One had extensive encephalomalacia and cerebral calcifications at presentation, and the other had multiple cardiac anomalies.
Conclusion
Our experience in the treatment of children with VGAM suggests that embolization during the first few weeks of life is unlikely to result in good outcomes and that endovascular treatment should be delayed, if possible, for several months. MR imaging and MR angiography have proved invaluable for the pretreatment analysis of these lesions and are the best imaging tools for the follow-up of incompletely eliminated lesions. We have found that pushable fibered coils are appropriate embolic agents to use in most cases, providing a good combination of control and permanence. Our embolization strategy is to occlude as many arterial feeders as is possible, avoiding venous occlusion until no other option is available. Our experience in one case suggests that there may be a role for the use of radiation therapy in cases in which rapidity of treatment is not essential. We think that ventricular drainage for treatment of hydrocephalus should be avoided until all embolization procedures are complete. Even at that point, it should be recognized that CSF diversion might exacerbate problems of venous insufficiency. Use of a clinical grading scale to determine aggressiveness and timing of treatment may help in avoiding fruitless treatment and aid in prognostication. A coherent policy on such treatment decisions should be developed by the entire group of physicians caring for these children, including neuroradiologists, neurosurgeons, cardiologists, and neonatologists.
Footnotes
Presented in part as an abstract at the 39th Annual Meeting of the American Society of Neuroradiology in Boston, on April 23, 2001.
References
- Received February 1, 2002.
- Accepted after revision July 10, 2002.
- Copyright © American Society of Neuroradiology