Abstract
BACKGROUND AND PURPOSE: The substantia innominata can be visualized on coronal thin-section T2-weighted MR images. The purpose of this study was to investigate the morphologic changes of the substantia innominata in normal aging by using MR imaging and to determine whether the changes in this structure on MR images were specific to Alzheimer disease (AD).
METHODS: The thickness of the substantia innominata was measured on the coronal T2-weighted image obtained through the anterior commissure in 39 healthy control subjects (age range, 25–86 y; mean age, 62 y); 39 patients with AD; and 36 patients with non-AD dementia, including vascular dementia, frontotemporal dementia, and Parkinson disease with dementia.
RESULTS: In the control subjects, the thickness of the substantia innominata significantly decreased with age. Compared with age-matched control subjects, both patients with AD and patients with non-AD dementia had significant atrophy of the substantia innominata. The thickness of the substantia innominata significantly correlated with scores from the Mini-Mental State Examination in patients with AD but not in patients with non-AD dementia.
CONCLUSION: MR analysis reveals age-related shrinkage of the substantia innominata. Atrophy of the substantia innominata, which reflects degeneration in the nucleus basalis of Meynert, is pronounced both in patients with AD and in those with non-AD dementia. MR imaging features in this structure may not be specific to AD.
The presence of a marked reduction and shrinkage of the large cells in the nucleus basalis of Meynert in the brains of patients with Alzheimer disease (AD) has been widely reported. Since the nucleus basalis of Meynert in the basal forebrain provides the major source of cholinergic input to the cerebral cortex, degeneration in the forebrain may represent an anatomic correlation of the loss of cholinergic markers in the cerebral cortex, and this may be involved in higher cortical dysfunction of AD (1, 2). The depiction of abnormalities in the nucleus basalis of Meynert on in vivo images appears to be necessary to understand the pathophysiology of AD.
In an MR imaging study in which postmortem specimens of human brain were compared, Sasaki et al (3) clarified the complex anatomy of the basal forebrain. The substantia innominata, in which the nucleus basalis of Meynert is located, can be readily identified as a narrow band below the globus pallidus on thin-section T2-weighted coronal MR images. In the present study, we investigated the morphologic changes of the substantia innominata in normal aging by using MR imaging. We compared the thickness of the substantia innominata in patients with AD with those in patients with non-AD dementia, including vascular dementia and other degenerative dementias, to determine whether MR imaging changes in this structure were specific to AD. Moreover, we studied the correlation of shrinkage in the substantia innominata with cognitive function both in patients with AD and in those with non-AD dementia.
Methods
Subjects
We examined 39 patients with AD (15 men, 24 women; age range, 67–88 y; mean age, 78 y), 36 patients with other types of dementia (16 men, 20 women; age range, 63–87 y; mean age, 77 y), and 39 healthy control subjects (16 men, 23 women; age range, 25–86 y; mean age, 62 y). To compare patients with AD and patients with other types of dementia, 21 age- and sex-matched elderly control subjects (nine men, 12 women; age range, 62–86 y; mean age, 76 y) were selected from the 39 healthy control subjects.
All patients with AD met the criteria for probable AD formulated by the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer’s Disease and Related Disorders Association, or NINCDS-ADRDA, Work Group (4). Patients with AD underwent general physical and neurologic examinations and extensive laboratory testing to exclude other potential causes of dementia. All had scores of less than 4 on the ischemic scale developed by Hachinski et al (5). Patients with evidence of stroke, as determined either with history or imaging findings, were excluded. Patients with AD were in the mild-to-moderate stages of the disease, with a mean Mini-Mental State Examination (MMSE) (6) score of 17.3 ± 4.4.
The group of subjects with other types of dementia (non-AD dementia) included 23 patients with probable vascular dementia (10 men, 13 women; mean age, 77 y), according to the criteria of the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences, or NINDS-AIREN, International Workshop (7); five patients with frontotemporal dementia (two men, three women; mean age, 73 y), whose diagnoses were made according to the criteria of the Lund and Manchester groups (8); and eight patients with idiopathic Parkinson disease with dementia (three men, five women; mean age, 78 y). All patients with a diagnosis of vascular dementia had multiple lacunar strokes of the basal gray matter and thalamus, in addition to periventricular and deep white matter lesions. They had scores of more than 7 on the ischemic scale developed by Hachinski et al (5). The mean MMSE scores of patients with vascular dementia, frontotemporal dementia, and Parkinson disease with dementia were 20.0 ± 3.5, 16.2 ± 6.4, and 19.8 ± 2.4, respectively. The dementia groups had no significant differences in age, sex, and MMSE scores.
The control subjects were cognitively healthy (mean MMSE score, 28.1 ± 1.2), were free of any neurologic or psychiatric illnesses, and had minimal white matter changes. None of the subjects, including those with AD, those with non-AD dementia, and the control group, had any lesions in the basal forebrain or in the tortuous vessels that compress the substantia innominata.
Written informed consent for the MR studies was obtained from all subjects, their spouses, or their family members.
MR Imaging
The subjects underwent imaging with a 1.5-T MR unit (Magnetom Symphony; Siemens Medical Systems, Erlangen, Germany) with a CP head array coil. Coronal T2-weighted images were obtained with a fast spin-echo sequence with parameters of 3000/100/3 (TR/TE/excitations). The section thickness was 3 mm, with an intersection gap of 0.9 mm. The matrix was 256 ± 231, and the field of view was 20 cm. Coronal images were perpendicular to the anterior commissure–posterior commissure line.
As Sasaki et al (3) described previously, the substantia innominata is best visualized in the plane through the anterior commissure on the coronal T2-weighted image. The substantia innominata has the same signal intensity as that of the gray matter and is identified as a narrow band below the subcommissural part of the globus pallidus because of the low signal intensity of the latter. The thickness of the substantia innominata, including the right and left sides, was measured on the coronal T2-weighted image through the anterior commissure by blinded a investigator (T.A.), with a threefold magnification for each image. The measurement was performed with a standard work console (Siemens Medical Systems). The contrast among the substantia innominata, globus pallidus, and CSF was carefully optimized on the cathode-ray terminal before the measurement was obtained. The distance between the lower margin of the low-signal-intensity area, which corresponded to the globus pallidus, and the surface of the substantia innominata was measured at the narrowest portion of the substantia innominata on the plane through the anterior commissure. Observer reliability was evaluated in 18 subjects with or without neurologic diseases. High intraobserver and interobserver reliability existed for the measurement of the thickness of the substantia innominata (r = .90 and r = 0.87, with 8.9% and 5.8% mean differences, respectively). The measurements of the thickness of the substantia innominata repeated after 3-month intervals differed by only 6.8% ± 2.9 (difference between the two measurements, n = 9).
Statistical Analysis
Values were expressed as the mean ± the SD. Between-group differences in the thickness of the substantia innominata were analyzed by using a one-way analysis of variance (ANOVA) with a post hoc Scheffé F test. Correlations between the thickness of the substantia innominata in healthy subjects and age and between the thickness and MMSE score were calculated by using Pearson correlation test. A P value of less than .05 was considered to indicate statistically significant difference.
Results
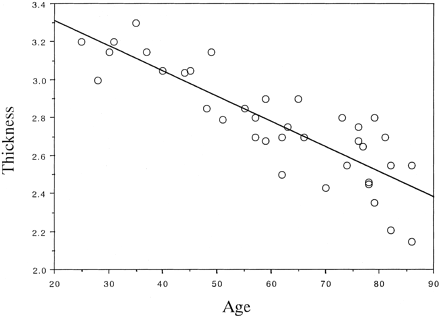
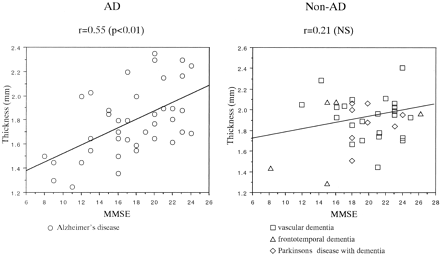
No statistical differences were found between the thickness of the substantia innominata on the right and left sides in any subject, including healthy control subjects, patients with AD, and those with non-AD dementia. The thickness on both sides correlated strongly (0.82 in control subjects, 0.86 in patients with AD, and 0.79 in those with non-AD dementia). Therefore, the average thickness, including that of the substantia innominata on the right and left sides, was calculated. In control subjects, the thickness of the substantia innominata significantly decreased with age (r = − .86, P < .0001), as shown in Figure 1. The thickness of the substantia innominata in patients with AD and in those with non-AD dementia was compared with that in 21 age- and sex-matched elderly control subjects. ANOVA revealed significant differences in the thickness of the substantia innominata between the groups (F = 33.94, P < .0001). The post hoc Scheffé F test revealed that the thickness in patients with AD and in those with vascular dementia, frontotemporal dementia, or Parkinson disease with dementia decreased more significantly than that in elderly control subjects (all P < .0001). However, no significant differences in thickness were found among the dementia groups (Table). The thickness of the substantia innominata significantly correlated with the MMSE score in patients with AD (r = .55, P < .01) but not in patients with non-AD dementia (r = .21, P > .05), including vascular dementia (r = ±.11, P < .05), frontotemporal dementia (r = .43, P > .05), and Parkinson disease with dementia (r = .01, P > .05) (Fig 2).
Plot shows the correlation between age and thickness of the substantia innominata in control subjects. The thickness of the substantia innominata significantly decreased with normal aging (y = 3.576 − 0.013x, r = −.86, P < .0001).
Plots show a significant correlation between MMSE scores and thickness of the substantia innominata in patients with AD but not in those with non-AD dementia. NS = not significant (P > .05).
Thickness of the substantia innominata in elderly control subjects and patients with dementia
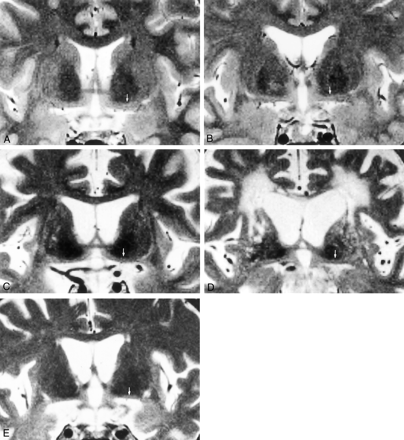
Figure 3 shows coronal T2-weighted images in a young healthy control subject; an elderly healthy control subject; and patients with AD, vascular dementia, or frontotemporal dementia.
Coronal T2-weighted MR images (3000/100/3; section thickness, 3 mm; intersection gap, 0.9 mm; matrix, 256 × 231; field of view, 20 cm) shows the substantia innominata in five subjects (arrows, A–E).
A, Image in a 37-year-old healthy female control subject.
B, Image in a 70-year-old healthy male control subject shows thinning of the substantia innominata.
C, Image in a 67-year-old man with AD shows prominent atrophy of the substantia innominata.
D, Image in a 74-year-old man with vascular dementia shows prominent atrophy of the substantia innominata.
E, Image in a 64-year-old woman with frontotemporal dementia shows prominent atrophy of the substantia innominata.
Discussion
We found that the thickness of the substantia innominata decreased with age and that the substantia innominata was atrophied not only in patients with AD but also in patients with non-AD dementia, compared with age-matched control subjects. Since the substantia innominata contains mainly the nucleus basalis of Meynert, atrophy of this structure is probably due to loss of neurons in the nucleus basalis of Meynert and a reduction in neuropils.
Several studies from McGeer et al (9), Mann et al (10), Lowes-Hummell et al (11), and De Lacalle et al (12) have revealed a significant age-dependent reduction in neurons in the nucleus basalis of Meynert. In their studies, subjects with a broad age range, including children, were included. In contrast, Chui et al (13) and Whitehouse et al (14) found that the numbers of neurons are largely stable in adults. Tissue-sampling differences could account for the disparity; the former groups counted all levels of the nucleus basalis of Meynert, and the other investigators counted only sections from a limited area. Moreover, in Chui et al’s study (13), nine of the 10 patients younger than 60 years had chronic alcoholism, and nearly all died of alcohol-related disease, which in itself can cause nerve cell loss in the nucleus basalis of Meynert. The relatively low nerve cell count in the young patients in this study may have caused the absence of an age-dependent decrease in nerve cells. In agreement with the former study’s findings, in which neurons in the nucleus basalis of Meynert were found to be vulnerable during normal aging, our results provide evidence of atrophy in the substantia innominata with aging.
Whitehouse et al (1, 2) reported a profound reduction in neurons from the nucleus basalis of Meynert in AD and suggested that degeneration of the nucleus basalis of Meynert was responsible for the cholinergic deficiency in the cerebral cortex. However, other investigators have found considerable variations in neuronal loss, ranging from 18% to 90% (1, 2, 9, 10, 15–21). This considerable variation in neuronal loss does not result from the use of different patient selection criteria and inconsistency in tissue processing but is mainly due to the counting procedures. On the other hand, several groups claimed that certain cholinergic neurons were shrunken rather than lost (15–20), suggesting retrograde degeneration in the nucleus basalis after damage of the cortex. Degeneration in the nucleus basalis of Meynert, regardless of whether it is neuronal loss or shrinkage, is likely to result in atrophy of the substantia innominata. In addition, significant neuronal loss has been demonstrated in neuropsychiatric diseases other than AD, such as Parkinson disease with and without dementia (21, 22), parkinsonism-dementia complex (23), Creutzfeldt-Jakob disease (24), progressive supranuclear palsy (25), Pick disease (26), Down syndrome (27), and Korsakoff disease (21). Moreover, Kato et al (28) reported that neurofibrillary tangle-bearing neurons were observed in the nucleus basalis of Meynert, ipsilateral to the cerebral infarcts, probably because of a retrograde reaction secondary to massive cerebral infarction. These findings suggest that degeneration in the nucleus basalis of Meynert can occur in a number of pathologically heterogeneous conditions. Our results, which revealed that the substantia innominata was atrophied not only in AD but also in non-AD dementia, were consistent with those of histopathologic studies of the nucleus basalis of Meynert. Thus, MR imaging depiction of the atrophy in this structure may not aid in improving specificity in the diagnosis of AD. In addition, choline acetyltransferase activity in cortical or subcortical areas of the brain is reported to be reduced in patients with dementia and Lewy bodies (29), Parkinson disease with dementia (30), Pick disease (31), vascular dementia (32), and other neurologic diseases. Therefore, the impairment of the cholinergic system may not be restricted to AD but may occur in other diseases characterized by the deterioration of higher cognitive functions.
However, the thickness of the substantia innominata was significantly correlated with the clinical severity assessed by using MMSE in AD but not in non-AD dementia. These results suggest that cognitive impairment is at least partly attributable to shrinkage of the substantia innominata, which indicates degeneration of ascending cholinergic neurons from the nucleus basalis of Meynert, in AD. Cholinergic dysfunction related to atrophy of the nucleus basalis of Meynert may play a different role in the cognitive functions of patients with AD and in those with non-AD dementia.
Some methodologic issues must be considered with regard to our study. First, because signal loss in the globus pallidus, caused by its magnetic susceptibility effect with the T2-weighted spin-echo technique, is substantial in elderly subjects (33) and patients with AD and other neurologic diseases (34), the thickness of the substantia innominata may be underestimated. However, signal loss in the globus pallidus is less prominent with the fast spin-echo technique, because of diminished sensitivity to susceptibility, than with the conventional spin-echo technique (35), and the contrast between the globus pallidus and the substantia innominata is sufficient to determine the boundary. Second, since the substantia innominata is a very thin structure and the rostrocaudal length is less than 20 mm, the imaging position and angulation of the imaging plane affects the thickness of the substantia innominata. Thus, in this study, the thickness of the substantia innominata was measured only in the plane through the anterior commissure. Imaging with higher spatial resolution and thinner sections would have little influence on the data.
Conclusion
Atrophy of the substantia innominata is not restricted to AD; it also occurs in non-AD dementia, including vascular dementia, frontotemporal dementia, and Parkinson disease with dementia. Our findings are, in general, consistent with those of previous postmortem human studies that include cell counting results in the nucleus basalis of Meynert. Atrophy of the substantia innominata may represent cholinergic system degeneration in the nucleus basalis of Meynert. MR analysis of this structure may be useful in understanding the pathophysiologic process of AD and other types of dementia.
Acknowledgments
We thank K. Sasaki and A. Katsuyama of the MR imaging team of Tokyo Medical University for their support and technical assistance. Also, we are grateful to J. Patrick Barron of the International Medical Communications Center of Tokyo Medical University for his review of the manuscript.
References
- Received July 2, 2001.
- Accepted after revision August 15, 2001.
- American Society of Neuroradiology