Abstract
BACKGROUND AND PURPOSE: Qualitative decreases in maternal brain size have been observed late in pregnancy. The aim of this study was to quantitatively evaluate changes to the maternal brain during and after healthy pregnancy and to compare these changes with those observed in cases of preeclampsia.
METHODS: Three-dimensional T1-weighted MR volume images were obtained in nine healthy participants before and after delivery. Additional images were obtained in some of these participants before pregnancy, during pregnancy, and within 52 weeks after delivery. Five women with preeclampsia were examined before delivery and 6 weeks after delivery. Three of these patients were examined within 52 weeks after delivery. Images were registered, and both brain and ventricular volumes were calculated by using a semiautomated computer program.
RESULTS: Both the healthy and preeclamptic groups had a reduction in brain size during pregnancy that was maximal at term and that reversed by 6 months after delivery. The ventricular size showed a corresponding increase in size during pregnancy and a decrease in size after delivery. In the preeclamptic patients, brain size was significantly smaller (P = .05) than in healthy participants, both before and after delivery.
CONCLUSION: The brain decreases in size during pregnancy and increases in size after delivery. The changes follow a consistent time course in each woman. The mechanism and physiologic importance of these findings are speculative at the present time.
During pregnancy, the pituitary gland increases in size (1–3), but the effect of pregnancy on the maternal brain as a whole is not known. Our aims in this study were to evaluate brain and ventricular size during and after healthy pregnancy with MR imaging and to compare any changes detected with those observed in patients with preeclampsia.
Methods
All MR images obtained in both healthy participants and preeclamptic patients were acquired with local research ethics committee permission and informed consent. Imaging was performed only after the first trimester of pregnancy, in accordance with guidelines published by the United Kingdom National Radiological Protection Board (4).
Healthy Group
A total of nine healthy pregnant women (mean age, 31.0 years; age range, 20–38 years) were included in the study. Two of the women had undergone imaging before becoming pregnant. Both of those women and two other healthy participants underwent imaging at various times from the 15th week of pregnancy to term (term is defined as the 37th–42nd week of pregnancy). Five other women in this group underwent MR imaging at term and 6 weeks after delivery. Eight of the nine women underwent additional follow-up MR imaging 24 weeks after delivery, and three were examined at 40 and 52 weeks after delivery (Table 1).
Number of subjects imaged in both groups
Preeclamptic Group
Five age-matched patients with preeclampsia (mean age, 32.2 years; age range, 29–38 years; one mild case and four severe cases) were examined. The criteria for the diagnosis of preeclampsia were as defined by the American College of Obstetrics and Gynecology (5). All five women were examined at 30th–39th weeks of pregnancy (≤ 2 weeks before delivery) and 6 weeks after delivery. Three of the patients with preeclampsia also underwent further MR imaging 24–52 weeks after delivery (Table 1).
MR Imaging
For all imaging performed during pregnancy, the abdomen and pelvis of each participant were positioned with the left side down (right side up) to avoid compression of the inferior vena cava. At each examination, 3D T1-weighted RF spoiled images (21/6 [TR/TE]; image matrix, 152 × 256 × 114; field of view, 25 cm; flip angle, 35°; section thickness, 1.6 mm) of the brain were acquired with a 1.0-T system. In each participant, subvoxel image registration (6) was used to accurately align the images on a common coordinate system (which was that of the images obtained at term in the healthy group and that of images obtained immediately before delivery in the preeclamptic group). To ensure the most accurate match, only the brain and not the surrounding tissue was used in the registration procedure. Isolation of the brain was performed by using a knowledge-based semiautomated segmentation program (7). Registered difference images were generated to illustrate the location and the type of change and to monitor their time course.
Qualitative Assessment
The registered subtraction images were visually assessed by two experienced observers. Changes detected in the brain were graded from 0 to 4, with 0 indicating no change and 4 indicating maximum change. A positive or negative sign also was used to indicate the direction of change (ie, increase or decrease in size, respectively); this addition resulted in a nine-point scale.
Quantitative Analysis
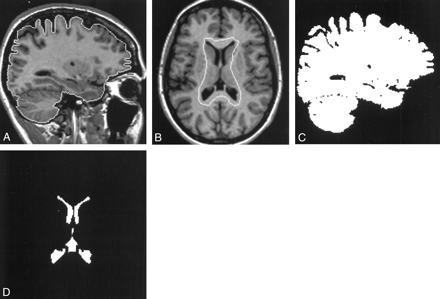
Volume measurements of both the brain and ventricles were obtained from the registered anatomic images by using a contour and thresholding technique (8). Examples of the contours generated are shown in Figure 1A and B. The contours were generated semiautomatically to surround but not touch the regions being measured and to exclude all other tissues of similar signal intensity. Threshold values for both brain and CSF were calculated automatically by means of histogram analysis, and binary images representing the pixel count for each region were generated (Fig 1C and D). The total voxel count from each image section was calculated, and volume measurements were obtained by adding the voxel count in all sections and multiplying this by the voxel size. The ventricular system measured included both lateral ventricles and the third ventricle but not the aqueduct or the fourth ventricle. The choroid plexuses were included in the voxel count.
Images illustrate the contour and thresholding technique.
A, Parasagittal 3D T1-weighted MR image of the brain with the brain contour.
B, Transverse 3D T1-weighted MR image of the brain showing the initial ventricular contour.
C, Binary image of the corresponding sections of the brain.
D, Final binary image of the ventricles.
Statistical Analysis
Paired t tests were performed to assess the significance of changes in the size of the brain and ventricles during and after pregnancy, as well as differences between the healthy and preeclamptic groups. The Spearman rank correlation coefficient was used to assess the relationship between the visual grades and the changes in volumes of the brain and ventricles.
Results
Clinical Features
No statistically significant difference in age was found between two groups (t = 0.37, df = 13, P > .7). The nine healthy participants had uncomplicated pregnancies. The five patients with preeclampsia had elevated blood pressure (>140/90 mm Hg) and notable proteinuria (>0.3 g/24 h), as measured on two separate occasions more than 4 hours apart. All patients except one had peripheral edema. The one patient who did not have edema had chronic renal failure and was treated with diuretics before, during, and after pregnancy. Four of the patients with preeclampsia received antihypertensive drugs. Three of these women were treated with dexamethasone (two 12-mg intramuscular injections administered 24 hours apart) before undergoing MR imaging. The steroid was administered to reduce the neonatal risk of respiratory distress syndrome. Three of the five patients with preeclampsia had headaches and blurred vision.
In this study, obstetric assessments were conducted at the time the images were acquired immediately before delivery in 13 of the 14 participants. All women had a normal fluid balance, as assessed by using hemoglobin and electrolyte concentrations. Two women with severe preeclampsia had albumin concentrations lower than those typical of a healthy pregnancy. Spinal anaesthesia was not contraindicated by clinical signs of increased intracranial pressure and was administered for delivery in three women with severe preeclampsia. No significant correlation was found between the presence of elevated blood pressure, proteinuria, peripheral edema, or CNS features and the magnitude of the changes in the brain or ventricles on the MR images.
Healthy Group
Visual Assessment of Brain and Ventricles.—
In the comparison of images obtained during pregnancy, the same pattern of change was observed on the registered subtraction images in each of the four participants examined during pregnancy. Low-signal-intensity borders that included both the cortex and lateral ventricles were present. This finding indicated a decrease in brain size (Fig 2C) and an increase in ventricular size (Fig 2C). For each participant, the greatest change was observed on the subtraction images generated from the images obtained earliest in the pregnancy minus those obtained at term.
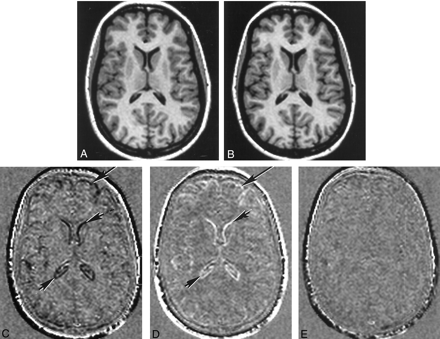
Registered and subtraction images.
A, Registered T1-weighted MR image obtained before conception.
B, Registered T1-weighted MR image obtained at term. Slight ventricular enlargement can be seen, compared with the image in A.
C, Subtraction image formed from the term image minus the preconception image. Ventricular enlargement is apparent as a dark line (small arrows). Decrease in the size of the brain is apparent at the external surface as dark lines (large arrow).
D, Subtraction image formed from the image obtained at 24 weeks minus the term image. An increase in the size of the ventricles between term and 24 weeks produces a white line (small arrows), and an increase in the size of the brain produces a white line at the external surface (large arrow).
E, Subtraction image formed from the image obtained at 24 weeks minus the preconception image. This largely featureless image is consistent with the brain and ventricles returning to their original preconception size at 24 weeks after delivery.
In the comparison of images obtained before (at term) and after delivery, the pattern of change was similar on the registered subtraction images in all nine healthy participants examined. High-signal-intensity borders were visible both in the region of the cortex and ventricles, showing that the brain had increased in size (Fig 2D) and that the ventricles had decreased in size (Fig 2D) after delivery. On the subtraction images made from later postdelivery images minus earlier postdelivery images, similar patterns of change again were seen, but to a lesser degree. The median grades for brain and ventricular change are shown in Table 2. The brain increased in size (range of medians, +1.8 to +2.0) after delivery, and the ventricles decreased in size (range of medians, −3.2 to −2.8) after delivery. In the two healthy participants who underwent imaging before conception, the registered subtraction images generated from the 24-week postdelivery images minus the preconception images showed little evidence of change in either the brain or the ventricles (Fig 2E). This finding was consistent with the brain and ventricles returning to their original sizes by the 24th week after delivery.
Median grades of change in brain and ventricles in size in both groups assessed on subtraction images
During pregnancy, the pituitary gland increased in size, was maximal at term, and decreased in size again as early as 6 weeks after delivery. This finding was present in all nine healthy participants and was best seen on the registered subtraction images.
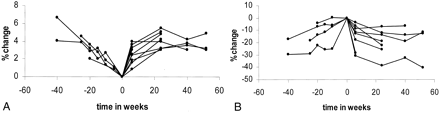
The percentage changes in brain size obtained at term and after delivery for all nine healthy participants are plotted in Figure 3A, and the absolute volume measurements are shown in Table 3. Figure 3A shows the same trend for all participants studied (ie, at each of the postdelivery time points, the brain had increased in size compared with its size at term, which was represented by 0). As early as 6 weeks after delivery, the increase in brain size was highly significant (P < .001). Also, a significant increase in size occurred at 24 weeks (P < .001) and on the later postdelivery images (P < .001). A significant increase in size was observed between 6 and 24 weeks (P = .007). The brains of the two healthy participants who were examined before conception appeared to return to their original size approximately 24 weeks after delivery (Fig 3A).
Healthy group.
A, Percentage changes in brain size before, during, and after pregnancy. The brain decreases in size until delivery and then increases in size again after delivery.
B, Percentage changes in ventricular size before, during, and after pregnancy. Ventricles increase in size until delivery and decrease in size after delivery.
Absolute brain volumes (cm3) before, during, and after pregnancy
The percentage change in ventricular volumes measured at term and after delivery in the healthy participants are shown in Figure 3B. The absolute changes in ventricular size are shown in Table 4. In all participants, a reduction in ventricular size was observed after delivery, compared with the size at term. The reduction in size was highly significant at 6 weeks (P < .001), 24 weeks (P < .001), and 40 weeks or later (P = .013). Also, a further significant reduction in size occurred between 6 weeks and the time of later postdelivery volume measurements (P < .024).
Absolute ventricular volumes (cm3) before, during, and after pregnancy
Quantitative Analysis.—
Both absolute volume measurements and percentage volume differences were calculated for the brain and ventricles of each participant. Percentage changes were calculated with reference to the last images acquired before delivery (baseline).
Quantitation of Images Obtained during Pregnancy.—
In the brain, the percentage changes for the four healthy participants are shown in Figure 3A, and the absolute volume measurements are shown in Table 3. The brain decreased in size during pregnancy in each participant, and the brain was smallest at term. In the two participants who were examined before conception, reductions of 4.1% (46.7 cm3) and 6.6% (80.2 cm3) were evident at term. In the other two participants who were examined from gestation of 15 or 19 weeks to term, the brain decreased in size by 4.5% (55.6 cm3) and 2% (19.3 cm3) at term.
In the ventricles, the percentage changes are shown in Figure 3B, and the absolute volume changes are shown in Table 4. During pregnancy, an increase in ventricular size was observed in all four participants, and the size was maximal at term. In the two participants who were examined before conception, increases in size of 29.5% (2.8 cm3) and 17.3% (3.7 cm3) were measured during pregnancy. In the two participants who were examined from a gestation of 15 or 19 weeks onward, the ventricles increased in size by 18.4% (3.3 cm3) and 4.1% (0.7 cm3) at term.
Preeclamptic Group
Visual Assessment of Brain and Ventricles.—
In the comparison of images obtained before and after delivery, the same pattern of change that was observed in the healthy group was present in the preeclamptic group. The brain increased in size after delivery (range of medians, +1.4 to +2.3), and the ventricles decreased in size (range of medians, −3.0 to −3.4). The pattern of change was the same in all patients.
Quantitative Assessment.—
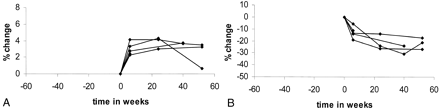
In the comparison of images before and after delivery in all five patients, the percentage changes in brain size are plotted in Figure 4A, and the absolute volume measurements in brain size are shown in Table 3. Figure 4A shows the same trend for all participants examined (ie, at each of the postdelivery time points, the size of the brain was increased, compared with the size before delivery). Again, the size of the brain significantly increased 6 weeks after delivery (P < .0002). Also, a significant increase in size was observed 24 weeks after delivery (P = .003) and at the later time points (P = .023). A significant increase in size was seen between the 6- and 24-week time points (P < .007).
Preeclamptic group.
A, Percentage changes in brain size before and after delivery. Brain increases in size after delivery. Exception is the last image of one patient who was treated with diuretics.
B, Percentage changes in ventricular size before and after delivery. Ventricles decrease in size after delivery.
The percentage changes in ventricular size before and after delivery in patients with preeclampsia are shown in Figure 4B. The absolute volume measurements are shown in Table 4. A reduction in ventricular size was observed after delivery, compared with the size before delivery, in all patients. The reduction in size was significant at 6 weeks (P < .03), at 24 weeks (P = .03), and on the late follow-up images (P = .05). Also, a significant reduction in size occurred between the 6-week and later postdelivery measurements (P < .024).
Comparison of Groups
Brain.—
A comparison of the healthy participants and patients with preeclampsia revealed that the brains in the patients with preeclampsia were significantly smaller than those of the healthy participants before delivery (P = .049), at 6 weeks after delivery (P = .05), at 24 weeks after delivery (P = .05), and at 40 weeks after delivery (P = .028). No difference was found at 52 weeks after delivery. In one of the patients with preeclampsia who was examined 52 weeks after delivery, the brain was smaller than it was at 24 weeks after delivery. The patient had chronic renal failure and was being treated with diuretics.
Ventricles.—
No significant difference in the size of the ventricles was found between the two groups at any of the time points that were compared. In the two participants who underwent imaging before conception, the ventricles returned to their original size between 10 and 24 weeks after delivery.
Discussion
In cases of both healthy pregnancy and preeclampsia, a highly significant reduction in brain size and increase in ventricular size were shown to occur during pregnancy, both visually and with volume measurements. The changes reversed after delivery. The decrease in brain size occurred with a concurrent increase in body circulatory and extracellular fluid volume and an increase in the size of other organs, such as the heart, kidney, and thyroid gland. The latter changes have been attributed to increase in vascular volume, cellular hypertrophy, and cellular hyperplasia (9) in response to hormonal and placental activity.
The decrease in brain size begins after placental implantation and is maximal at term, reversing slowly thereafter. In speculating about the cause, this time course must be taken into account. One possible explanation for the changes is that some aspect of hormonal, vascular, or metabolic alterations associated with placental implantation generates them. However, direct placental control of the process does not persist into the postpartum period, when the changes to brain were still evident. In contrast, metabolic changes occurring during pregnancy that cause cellular atrophy may continue for some months after delivery, because metabolic substrates or nutrients initially provided for the fetus may be diverted to the neonate during lactation or to maternal tissue reparation after delivery.
Overt nutritional deficiencies are not common during pregnancy, but the function of maternal tissue enzymes may depend on elements or chemicals, such as iron or folate, that are present in less than optimal levels, compared with those of the general population (10, 11). The levels of sex steroid hormones generated by the placenta and pituitary gland that reach a peak at delivery also may influence cellular size by controlling intracellular genomic activity. Macroscopically, a decrease in brain size has been observed when large doses of exogenous steroids are administered (12). Biochemical changes in CSF that typically cause a notable decrease in CSF density at term (13) and after delivery (14) present further evidence of substantial alterations in metabolic activity. Thus, multiple factors related to hormonal and metabolic effects could contribute to the volumetric changes of the brain during pregnancy.
Increases in levels of serum lipids such as triglycerides, cholesterol, low-density cholesterol, and high-density cholesterol are present during the second and third trimesters of pregnancy. Some have hypothesized that the changes in phospholipid content of brain membranes could reduce brain size (15). During pregnancy, the fetus can scavenge essential fatty acids and alter the make-up of maternal phospholipid membranes and, hence, potentially change brain morphology. Further studies of maternal phospholipid levels are needed to support this theory. Interestingly, the persistence of the brain changes after delivery was not related to lactation; the time course in all the women was similar, and some did not breast feed.
The brain was significantly smaller in the preeclamptic group, as measured on the images obtained before delivery and at 6, 24, and 40 weeks after delivery. Whether this was the result of the disease process itself or another cause, such as medical treatment, is not known. However, that preeclampsia is associated with reduced intravascular fluid expansion (16) is known; this may partly explain the smaller brain size. In this study, four of the five patients with preeclampsia were being treated with antihypertensive drugs, and three received steroids. Additionally, one of the patients with preeclampsia had chronic renal failure and received regular doses of diuretics. The brain of this patient decreased in size between the time of her 6- and 52-week post delivery imaging examinations, unlike every other participant examined. Also, the change in ventricular size was greatest in this patient; again, this may have been linked to her diuretic treatment. The women with preeclampsia were not homogeneous with respect to the duration of their pregnancies, and three received a short course of steroids. Steroids may decrease the size of the brain when administered long term (12), but the dosage was much less than that usually associated with brain atrophy. Additionally, the time course of the changes in the three patients who received steroids followed that of the two patients who did not receive steroids; this finding suggested that the drugs might not have had a considerable role. In any case, the role of steroids in inducing (reversible) cerebral atrophy is somewhat controversial (17, 18).
Preeclampsia is thought to be secondary to an interaction between placental and maternal factors (19). Abnormal lipid metabolism and oxidative stress may induce widespread endothelial dysfunction (20, 21) and abnormal vascular reactivity. These effects may be mediated by abnormal concentrations of circulating (eg, peptides) or local (eg, nitric oxide system) vasoactive agents (22, 23), leading to intracranial arterial vasoconstriction, which is a feature of untreated preeclampsia. This vasoconstriction may be sufficient to further reduce the typically observed change in brain size at term. However, several factors, such as treatment to induce vasodilation in the mother and the administration of steroid prophylaxis for fetal lung maturation, may have altered the natural disease process in the women with preeclampsia. The pronounced difference between healthy women and those with preeclampsia suggests that either the underlying mechanisms for the changes were more extreme or that other factors were influencing the results. For example, despite antihypertensive treatment in all women except those with mild preeclampsia, the effect of the vasodilators might not have been optimal or it may have began too soon before imaging to have a detectable effect. Further support for our observations can be found in the report of a brain CT study in which ventricular enlargement was present in nine of 44 women with eclampsia (24). These findings are in contrast with those of other studies showing that cerebral edema occurs in association with eclampsia (25, 26). Possibly, the cerebral edema found in these latter studies may represent a clinical extreme.
Preeclampsia is associated with endothelial dysfunction, and increased permeability across the vascular wall possibly can contribute to cerebral edema in severe cases (27). The acute tension in cerebral structures, such as thin-walled blood vessels, that may accompany the typical reduction in brain size could be rapidly converted into a shearing force that can induce hemorrhage and other vascular events. One explanation for the cause of the convulsions themselves may be that forces acting on the blood supply to particular brain regions could generate focal lesions as a result of compromised regional blood flow.
The reduction in brain size may have implications for the management of some pregnancy-related diseases. The present forms of treatment for preeclampsia and eclampsia include fluid restriction and the use of vasoactive drugs, such as diuretics, to help control or reduce generalized edema. With the findings of reduced brain volume during both pregnancy and early postpartum, as shown in this study, these treatments may negatively affect brain volume changes. Possibly, they could lead to a reduction in cerebral blood flow and/or volume during pregnancy and, hence, exacerbate the risk of ischemia and infarction, both of which are associated with preeclampsia and eclampsia (28, 29).
Conclusion
These findings show that the maternal brain decreases in size during healthy pregnancy and increases in size after delivery. The lateral ventricles increase in size and decrease after delivery. The same pattern is seen in patients with preeclampsia. The precise mechanism and physiologic importance of the changes is not known at the present time.
Acknowledgments
We acknowledge support from Marconi Medical Systems and the Obstetric Anaesthetists’ Association.
References
- Received February 23, 2001.
- Accepted after revision August 1, 2001.
- American Society of Neuroradiology